登録情報 データベース : EMDB / ID : EMD-32823タイトル Prefoldin-tubulin-TRiC complex A differently local sharpened main map for the refinement 複合体 : Ternary complex of TRiC/CCT, beta-tubulin, prefoldin complexリガンド : x 1種 / 機能・相同性 分子機能 ドメイン・相同性 構成要素
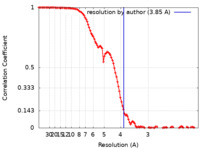
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / 生物種 Homo sapiens (ヒト)手法 / / 解像度 : 3.85 Å Roh SH / Park J 資金援助 Organization Grant number 国 National Research Foundation (NRF, Korea)
ジャーナル : Cell / 年 : 2022タイトル : Structural visualization of the tubulin folding pathway directed by human chaperonin TRiC/CCT.著者 : Daniel Gestaut / Yanyan Zhao / Junsun Park / Boxue Ma / Alexander Leitner / Miranda Collier / Grigore Pintilie / Soung-Hun Roh / Wah Chiu / Judith Frydman / 要旨 : The ATP-dependent ring-shaped chaperonin TRiC/CCT is essential for cellular proteostasis. To uncover why some eukaryotic proteins can only fold with TRiC assistance, we reconstituted the folding of ... The ATP-dependent ring-shaped chaperonin TRiC/CCT is essential for cellular proteostasis. To uncover why some eukaryotic proteins can only fold with TRiC assistance, we reconstituted the folding of β-tubulin using human prefoldin and TRiC. We find unstructured β-tubulin is delivered by prefoldin to the open TRiC chamber followed by ATP-dependent chamber closure. Cryo-EM resolves four near-atomic-resolution structures containing progressively folded β-tubulin intermediates within the closed TRiC chamber, culminating in native tubulin. This substrate folding pathway appears closely guided by site-specific interactions with conserved regions in the TRiC chamber. Initial electrostatic interactions between the TRiC interior wall and both the folded tubulin N domain and its C-terminal E-hook tail establish the native substrate topology, thus enabling C-domain folding. Intrinsically disordered CCT C termini within the chamber promote subsequent folding of tubulin's core and middle domains and GTP-binding. Thus, TRiC's chamber provides chemical and topological directives that shape the folding landscape of its obligate substrates. 履歴 登録 2022年2月7日 - ヘッダ(付随情報) 公開 2022年12月21日 - マップ公開 2022年12月21日 - 更新 2024年6月26日 - 現状 2024年6月26日 処理サイト : PDBj / 状態 : 公開
すべて表示 表示を減らす
 データを開く
データを開く 基本情報
基本情報
 マップデータ
マップデータ 試料
試料 キーワード
キーワード 機能・相同性情報
機能・相同性情報 Homo sapiens (ヒト)
Homo sapiens (ヒト) データ登録者
データ登録者 韓国, 1件
韓国, 1件  引用
引用 ジャーナル: Cell / 年: 2022
ジャーナル: Cell / 年: 2022


 構造の表示
構造の表示 ダウンロードとリンク
ダウンロードとリンク emd_32823.map.gz
emd_32823.map.gz EMDBマップデータ形式
EMDBマップデータ形式 emd-32823-v30.xml
emd-32823-v30.xml emd-32823.xml
emd-32823.xml EMDBヘッダ
EMDBヘッダ emd_32823_fsc.xml
emd_32823_fsc.xml FSCデータファイル
FSCデータファイル emd_32823.png
emd_32823.png emd-32823.cif.gz
emd-32823.cif.gz emd_32823_additional_1.map.gz
emd_32823_additional_1.map.gz emd_32823_half_map_1.map.gz
emd_32823_half_map_1.map.gz emd_32823_half_map_2.map.gz
emd_32823_half_map_2.map.gz http://ftp.pdbj.org/pub/emdb/structures/EMD-32823
http://ftp.pdbj.org/pub/emdb/structures/EMD-32823 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32823
ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32823 リンク
リンク EMDB (EBI/PDBe) /
EMDB (EBI/PDBe) /  EMDataResource
EMDataResource マップ
マップ ダウンロード / ファイル: emd_32823.map.gz / 形式: CCP4 / 大きさ: 125 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES)
ダウンロード / ファイル: emd_32823.map.gz / 形式: CCP4 / 大きさ: 125 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) 試料の構成要素
試料の構成要素 解析
解析 試料調製
試料調製 電子顕微鏡法
電子顕微鏡法 FIELD EMISSION GUN
FIELD EMISSION GUN
 ムービー
ムービー コントローラー
コントローラー

























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)






































 Trichoplusia ni (イラクサキンウワバ)
Trichoplusia ni (イラクサキンウワバ)