+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Prefoldin-tubulin-TRiC complex | |||||||||
 Map data Map data | A differently local sharpened main map for the refinement | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | chapronin complex / CHAPERONE | |||||||||
| Function / homology |  Function and homology information Function and homology informationRNA polymerase I assembly / RNA polymerase III assembly / prefoldin complex / positive regulation of cytoskeleton organization / zona pellucida receptor complex / scaRNA localization to Cajal body / chaperone mediated protein folding independent of cofactor / positive regulation of establishment of protein localization to telomere / positive regulation of protein localization to Cajal body / RNA polymerase II core complex assembly ...RNA polymerase I assembly / RNA polymerase III assembly / prefoldin complex / positive regulation of cytoskeleton organization / zona pellucida receptor complex / scaRNA localization to Cajal body / chaperone mediated protein folding independent of cofactor / positive regulation of establishment of protein localization to telomere / positive regulation of protein localization to Cajal body / RNA polymerase II core complex assembly / tubulin complex assembly / BBSome-mediated cargo-targeting to cilium / chaperonin-containing T-complex / RPAP3/R2TP/prefoldin-like complex / binding of sperm to zona pellucida / positive regulation of telomerase RNA localization to Cajal body / Folding of actin by CCT/TriC / Formation of tubulin folding intermediates by CCT/TriC / Prefoldin mediated transfer of substrate to CCT/TriC / negative regulation of amyloid fibril formation / protein folding chaperone complex / RHOBTB1 GTPase cycle / intermediate filament cytoskeleton / WD40-repeat domain binding / pericentriolar material / beta-tubulin binding / : / Association of TriC/CCT with target proteins during biosynthesis / microtubule-based process / chaperone-mediated protein complex assembly / RHOBTB2 GTPase cycle / heterochromatin / chaperone-mediated protein folding / protein folding chaperone / positive regulation of telomere maintenance via telomerase / Gene and protein expression by JAK-STAT signaling after Interleukin-12 stimulation / tubulin binding / acrosomal vesicle / cell projection / mRNA 3'-UTR binding / ATP-dependent protein folding chaperone / response to virus / negative regulation of canonical Wnt signaling pathway / cilium / mRNA 5'-UTR binding / transcription corepressor activity / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / azurophil granule lumen / G-protein beta-subunit binding / unfolded protein binding / melanosome / protein folding / protein-folding chaperone binding / retina development in camera-type eye / amyloid-beta binding / cell body / secretory granule lumen / ficolin-1-rich granule lumen / microtubule / cytoskeleton / protein stabilization / cadherin binding / intracellular membrane-bounded organelle / negative regulation of DNA-templated transcription / centrosome / ubiquitin protein ligase binding / Neutrophil degranulation / regulation of DNA-templated transcription / Golgi apparatus / ATP hydrolysis activity / mitochondrion / RNA binding / extracellular exosome / extracellular region / nucleoplasm / ATP binding / nucleus / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
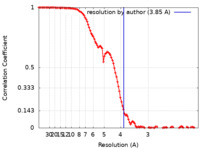
| Method | single particle reconstruction / cryo EM / Resolution: 3.85 Å | |||||||||
 Authors Authors | Roh SH / Park J | |||||||||
| Funding support |  Korea, Republic Of, 1 items Korea, Republic Of, 1 items
| |||||||||
 Citation Citation |  Journal: Cell / Year: 2022 Journal: Cell / Year: 2022Title: Structural visualization of the tubulin folding pathway directed by human chaperonin TRiC/CCT. Authors: Daniel Gestaut / Yanyan Zhao / Junsun Park / Boxue Ma / Alexander Leitner / Miranda Collier / Grigore Pintilie / Soung-Hun Roh / Wah Chiu / Judith Frydman /    Abstract: The ATP-dependent ring-shaped chaperonin TRiC/CCT is essential for cellular proteostasis. To uncover why some eukaryotic proteins can only fold with TRiC assistance, we reconstituted the folding of ...The ATP-dependent ring-shaped chaperonin TRiC/CCT is essential for cellular proteostasis. To uncover why some eukaryotic proteins can only fold with TRiC assistance, we reconstituted the folding of β-tubulin using human prefoldin and TRiC. We find unstructured β-tubulin is delivered by prefoldin to the open TRiC chamber followed by ATP-dependent chamber closure. Cryo-EM resolves four near-atomic-resolution structures containing progressively folded β-tubulin intermediates within the closed TRiC chamber, culminating in native tubulin. This substrate folding pathway appears closely guided by site-specific interactions with conserved regions in the TRiC chamber. Initial electrostatic interactions between the TRiC interior wall and both the folded tubulin N domain and its C-terminal E-hook tail establish the native substrate topology, thus enabling C-domain folding. Intrinsically disordered CCT C termini within the chamber promote subsequent folding of tubulin's core and middle domains and GTP-binding. Thus, TRiC's chamber provides chemical and topological directives that shape the folding landscape of its obligate substrates. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_32823.map.gz emd_32823.map.gz | 118 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-32823-v30.xml emd-32823-v30.xml emd-32823.xml emd-32823.xml | 38 KB 38 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_32823_fsc.xml emd_32823_fsc.xml | 11.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_32823.png emd_32823.png | 87.7 KB | ||
| Filedesc metadata |  emd-32823.cif.gz emd-32823.cif.gz | 10.2 KB | ||
| Others |  emd_32823_additional_1.map.gz emd_32823_additional_1.map.gz emd_32823_half_map_1.map.gz emd_32823_half_map_1.map.gz emd_32823_half_map_2.map.gz emd_32823_half_map_2.map.gz | 118 MB 115.9 MB 115.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-32823 http://ftp.pdbj.org/pub/emdb/structures/EMD-32823 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32823 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32823 | HTTPS FTP |
-Validation report
| Summary document |  emd_32823_validation.pdf.gz emd_32823_validation.pdf.gz | 1 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_32823_full_validation.pdf.gz emd_32823_full_validation.pdf.gz | 1 MB | Display | |
| Data in XML |  emd_32823_validation.xml.gz emd_32823_validation.xml.gz | 19.1 KB | Display | |
| Data in CIF |  emd_32823_validation.cif.gz emd_32823_validation.cif.gz | 24.5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32823 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32823 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32823 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32823 | HTTPS FTP |
-Related structure data
| Related structure data |  7wu7MC  7trgC  7ttnC  7tttC  7tubC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_32823.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_32823.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | A differently local sharpened main map for the refinement | ||||||||||||||||||||||||||||||||||||
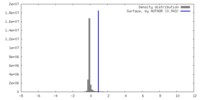
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.02 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: A B-factor sharpened map
| File | emd_32823_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | A B-factor sharpened map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: A half map
| File | emd_32823_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | A half map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: A half map
| File | emd_32823_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | A half map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : Ternary complex of TRiC/CCT, beta-tubulin, prefoldin complex
+Supramolecule #1: Ternary complex of TRiC/CCT, beta-tubulin, prefoldin complex
+Macromolecule #1: Prefoldin subunit 1
+Macromolecule #2: Prefoldin subunit 2
+Macromolecule #3: Prefoldin subunit 3
+Macromolecule #4: Prefoldin subunit 4
+Macromolecule #5: Prefoldin subunit 5
+Macromolecule #6: Prefoldin subunit 6
+Macromolecule #7: T-complex protein 1 subunit alpha
+Macromolecule #8: T-complex protein 1 subunit beta
+Macromolecule #9: T-complex protein 1 subunit gamma
+Macromolecule #10: T-complex protein 1 subunit delta
+Macromolecule #11: T-complex protein 1 subunit epsilon
+Macromolecule #12: T-complex protein 1 subunit zeta
+Macromolecule #13: T-complex protein 1 subunit eta
+Macromolecule #14: T-complex protein 1 subunit theta
+Macromolecule #15: ADENOSINE-5'-DIPHOSPHATE
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | 3D array |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 Component:
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Number real images: 11796 / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 3.5 µm / Nominal defocus min: 0.5 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller




















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)






































 Trichoplusia ni (cabbage looper)
Trichoplusia ni (cabbage looper)