[English] 日本語
 Yorodumi
Yorodumi- EMDB-32100: STRUCTURE OF PHOTOSYNTHETIC LH1-RC SUPER-COMPLEX OF Allochromatiu... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | STRUCTURE OF PHOTOSYNTHETIC LH1-RC SUPER-COMPLEX OF Allochromatium tepidum | |||||||||||||||||||||
 Map data Map data | ||||||||||||||||||||||
 Sample Sample |
| |||||||||||||||||||||
 Keywords Keywords | LH1-RC COMPLEX / PHOTOSYNTHESIS / PURPLE BACTERIA | |||||||||||||||||||||
| Biological species |  Allochromatium tepidum (bacteria) Allochromatium tepidum (bacteria) | |||||||||||||||||||||
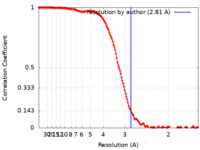
| Method | single particle reconstruction / cryo EM / Resolution: 2.81 Å | |||||||||||||||||||||
 Authors Authors | Tani K / Kobayashi K | |||||||||||||||||||||
| Funding support |  Japan, 6 items Japan, 6 items
| |||||||||||||||||||||
 Citation Citation |  Journal: J Biol Chem / Year: 2022 Journal: J Biol Chem / Year: 2022Title: A Ca-binding motif underlies the unusual properties of certain photosynthetic bacterial core light-harvesting complexes. Authors: Kazutoshi Tani / Kazumi Kobayashi / Naoki Hosogi / Xuan-Cheng Ji / Sakiko Nagashima / Kenji V P Nagashima / Airi Izumida / Kazuhito Inoue / Yusuke Tsukatani / Ryo Kanno / Malgorzata Hall / ...Authors: Kazutoshi Tani / Kazumi Kobayashi / Naoki Hosogi / Xuan-Cheng Ji / Sakiko Nagashima / Kenji V P Nagashima / Airi Izumida / Kazuhito Inoue / Yusuke Tsukatani / Ryo Kanno / Malgorzata Hall / Long-Jiang Yu / Isamu Ishikawa / Yoshihiro Okura / Michael T Madigan / Akira Mizoguchi / Bruno M Humbel / Yukihiro Kimura / Zheng-Yu Wang-Otomo /    Abstract: The mildly thermophilic purple phototrophic bacterium Allochromatium tepidum provides a unique model for investigating various intermediate phenotypes observed between those of thermophilic and ...The mildly thermophilic purple phototrophic bacterium Allochromatium tepidum provides a unique model for investigating various intermediate phenotypes observed between those of thermophilic and mesophilic counterparts. The core light-harvesting (LH1) complex from A. tepidum exhibits an absorption maximum at 890 nm and mildly enhanced thermostability, both of which are Ca-dependent. However, it is unknown what structural determinants might contribute to these properties. Here, we present a cryo-EM structure of the reaction center-associated LH1 complex at 2.81 Å resolution, in which we identify multiple pigment-binding α- and β-polypeptides within an LH1 ring. Of the 16 α-polypeptides, we show that six (α1) bind Ca along with β1- or β3-polypeptides to form the Ca-binding sites. This structure differs from that of fully Ca-bound LH1 from Thermochromatium tepidum, enabling determination of the minimum structural requirements for Ca-binding. We also identified three amino acids (Trp44, Asp47, and Ile49) in the C-terminal region of the A. tepidum α1-polypeptide that ligate each Ca ion, forming a Ca-binding WxxDxI motif that is conserved in all Ca-bound LH1 α-polypeptides from other species with reported structures. The partial Ca-bound structure further explains the unusual phenotypic properties observed for this bacterium in terms of its Ca-requirements for thermostability, spectroscopy, and phototrophic growth, and supports the hypothesis that A. tepidum may represent a "transitional" species between mesophilic and thermophilic purple sulfur bacteria. The characteristic arrangement of multiple αβ-polypeptides also suggests a mechanism of molecular recognition in the expression and/or assembly of the LH1 complex that could be regulated through interactions with reaction center subunits. | |||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_32100.map.gz emd_32100.map.gz | 228.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-32100-v30.xml emd-32100-v30.xml emd-32100.xml emd-32100.xml | 30.4 KB 30.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_32100_fsc.xml emd_32100_fsc.xml | 14.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_32100.png emd_32100.png | 183.1 KB | ||
| Filedesc metadata |  emd-32100.cif.gz emd-32100.cif.gz | 8.5 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-32100 http://ftp.pdbj.org/pub/emdb/structures/EMD-32100 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32100 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32100 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_32100.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_32100.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.814 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
+Entire : Photosynthetic LH1-RC complex from the purple phototrophic bacter...
+Supramolecule #1: Photosynthetic LH1-RC complex from the purple phototrophic bacter...
+Macromolecule #1: Photosynthetic reaction center cytochrome c subunit
+Macromolecule #2: Photosynthetic reaction center L subunit
+Macromolecule #3: Photosynthetic reaction center M subunit
+Macromolecule #4: Photosynthetic reaction center H subunit
+Macromolecule #5: Light-harvesting protein LH1 alpha2
+Macromolecule #6: Light-harvesting protein LH1 beta1
+Macromolecule #7: Light-harvesting protein LH1 alpha1
+Macromolecule #8: Light-harvesting protein LH1 beta3
+Macromolecule #9: Light-harvesting protein LH1 alpha3
+Macromolecule #10: HEME C
+Macromolecule #11: MAGNESIUM ION
+Macromolecule #12: DIACYL GLYCEROL
+Macromolecule #13: PALMITIC ACID
+Macromolecule #14: (1R)-2-{[{[(2S)-2,3-DIHYDROXYPROPYL]OXY}(HYDROXY)PHOSPHORYL]OXY}-...
+Macromolecule #15: BACTERIOCHLOROPHYLL A
+Macromolecule #16: BACTERIOPHEOPHYTIN A
+Macromolecule #17: Ubiquinone-8
+Macromolecule #18: CARDIOLIPIN
+Macromolecule #19: FE (III) ION
+Macromolecule #20: MENAQUINONE 8
+Macromolecule #21: SPIRILLOXANTHIN
+Macromolecule #22: DODECYL-BETA-D-MALTOSIDE
+Macromolecule #23: CALCIUM ION
+Macromolecule #24: LAURYL DIMETHYLAMINE-N-OXIDE
+Macromolecule #25: water
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 3.0 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 90 % / Chamber temperature: 277 K / Instrument: LEICA EM GP |
| Details | This sample was monodisperse. |
- Electron microscopy
Electron microscopy
| Microscope | JEOL CRYO ARM 300 |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Detector mode: COUNTING / Average exposure time: 1.5 sec. / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Sample stage | Specimen holder model: JEOL / Cooling holder cryogen: NITROGEN |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Source name: PDB / Chain - Initial model type: experimental model |
|---|---|
| Refinement | Space: REAL / Protocol: RIGID BODY FIT / Overall B value: 62 / Target criteria: Correlation coefficient |
| Output model |  PDB-7vrj: |
 Movie
Movie Controller
Controller



 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)