[English] 日本語
 Yorodumi
Yorodumi- EMDB-30275: COVID-19 RNA-dependent RNA polymerase pre-translocated catalytic ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-30275 | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | COVID-19 RNA-dependent RNA polymerase pre-translocated catalytic complex | ||||||||||||||||||||||||
 Map data Map data | |||||||||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||||||||
 Keywords Keywords | COVID-19 / 2019-nCoV / SARS-CoV-2 / Virus / RdRp / nsp12 / nsp7 / nsp8 / RTC / cryo-EM / Viral protein / RNA polymerase / drug target / antiviral / pre-translocated catalytic complex / VIRAL PROTEIN-RNA complex | ||||||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationprotein guanylyltransferase activity / RNA endonuclease activity producing 3'-phosphomonoesters, hydrolytic mechanism / mRNA guanylyltransferase activity / 5'-3' RNA helicase activity / Lyases; Phosphorus-oxygen lyases / Assembly of the SARS-CoV-2 Replication-Transcription Complex (RTC) / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of TBK1 activity / Maturation of replicase proteins / TRAF3-dependent IRF activation pathway / ISG15-specific peptidase activity ...protein guanylyltransferase activity / RNA endonuclease activity producing 3'-phosphomonoesters, hydrolytic mechanism / mRNA guanylyltransferase activity / 5'-3' RNA helicase activity / Lyases; Phosphorus-oxygen lyases / Assembly of the SARS-CoV-2 Replication-Transcription Complex (RTC) / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of TBK1 activity / Maturation of replicase proteins / TRAF3-dependent IRF activation pathway / ISG15-specific peptidase activity / Transcription of SARS-CoV-2 sgRNAs / snRNP Assembly / Translation of Replicase and Assembly of the Replication Transcription Complex / Replication of the SARS-CoV-2 genome / Hydrolases; Acting on ester bonds; Exoribonucleases producing 5'-phosphomonoesters / host cell endoplasmic reticulum-Golgi intermediate compartment / double membrane vesicle viral factory outer membrane / 3'-5'-RNA exonuclease activity / SARS coronavirus main proteinase / 5'-3' DNA helicase activity / host cell endosome / symbiont-mediated degradation of host mRNA / mRNA guanylyltransferase / symbiont-mediated suppression of host ISG15-protein conjugation / symbiont-mediated suppression of host toll-like receptor signaling pathway / G-quadruplex RNA binding / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of IRF3 activity / omega peptidase activity / SARS-CoV-2 modulates host translation machinery / mRNA (guanine-N7)-methyltransferase / methyltransferase cap1 / host cell Golgi apparatus / symbiont-mediated suppression of host NF-kappaB cascade / symbiont-mediated perturbation of host ubiquitin-like protein modification / DNA helicase / single-stranded 3'-5' DNA helicase activity / double-stranded DNA helicase activity / forked DNA-dependent helicase activity / four-way junction helicase activity / methyltransferase cap1 activity / ubiquitinyl hydrolase 1 / mRNA 5'-cap (guanine-N7-)-methyltransferase activity / cysteine-type deubiquitinase activity / Hydrolases; Acting on peptide bonds (peptidases); Cysteine endopeptidases / single-stranded RNA binding / host cell perinuclear region of cytoplasm / regulation of autophagy / viral protein processing / lyase activity / host cell endoplasmic reticulum membrane / RNA helicase / symbiont-mediated suppression of host type I interferon-mediated signaling pathway / symbiont-mediated suppression of host gene expression / copper ion binding / viral translational frameshifting / symbiont-mediated activation of host autophagy / RNA-directed RNA polymerase / cysteine-type endopeptidase activity / viral RNA genome replication / RNA-directed RNA polymerase activity / DNA-templated transcription / lipid binding / host cell nucleus / SARS-CoV-2 activates/modulates innate and adaptive immune responses / ATP hydrolysis activity / proteolysis / RNA binding / zinc ion binding / ATP binding / membrane Similarity search - Function | ||||||||||||||||||||||||
| Biological species |    Foot-and-mouth disease virus Foot-and-mouth disease virus | ||||||||||||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.93 Å | ||||||||||||||||||||||||
 Authors Authors | Wang Q / Gao Y / Ji W / Mu A / Rao Z | ||||||||||||||||||||||||
| Funding support |  China, 7 items China, 7 items
| ||||||||||||||||||||||||
 Citation Citation |  Journal: Cell / Year: 2020 Journal: Cell / Year: 2020Title: Structural Basis for RNA Replication by the SARS-CoV-2 Polymerase. Authors: Quan Wang / Jiqin Wu / Haofeng Wang / Yan Gao / Qiaojie Liu / An Mu / Wenxin Ji / Liming Yan / Yan Zhu / Chen Zhu / Xiang Fang / Xiaobao Yang / Yucen Huang / Hailong Gao / Fengjiang Liu / Ji ...Authors: Quan Wang / Jiqin Wu / Haofeng Wang / Yan Gao / Qiaojie Liu / An Mu / Wenxin Ji / Liming Yan / Yan Zhu / Chen Zhu / Xiang Fang / Xiaobao Yang / Yucen Huang / Hailong Gao / Fengjiang Liu / Ji Ge / Qianqian Sun / Xiuna Yang / Wenqing Xu / Zhijie Liu / Haitao Yang / Zhiyong Lou / Biao Jiang / Luke W Guddat / Peng Gong / Zihe Rao /   Abstract: Nucleotide analog inhibitors, including broad-spectrum remdesivir and favipiravir, have shown promise in in vitro assays and some clinical studies for COVID-19 treatment, this despite an incomplete ...Nucleotide analog inhibitors, including broad-spectrum remdesivir and favipiravir, have shown promise in in vitro assays and some clinical studies for COVID-19 treatment, this despite an incomplete mechanistic understanding of the viral RNA-dependent RNA polymerase nsp12 drug interactions. Here, we examine the molecular basis of SARS-CoV-2 RNA replication by determining the cryo-EM structures of the stalled pre- and post- translocated polymerase complexes. Compared with the apo complex, the structures show notable structural rearrangements happening to nsp12 and its co-factors nsp7 and nsp8 to accommodate the nucleic acid, whereas there are highly conserved residues in nsp12, positioning the template and primer for an in-line attack on the incoming nucleotide. Furthermore, we investigate the inhibition mechanism of the triphosphate metabolite of remdesivir through structural and kinetic analyses. A transition model from the nsp7-nsp8 hexadecameric primase complex to the nsp12-nsp7-nsp8 polymerase complex is also proposed to provide clues for the understanding of the coronavirus transcription and replication machinery. | ||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_30275.map.gz emd_30275.map.gz | 117.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-30275-v30.xml emd-30275-v30.xml emd-30275.xml emd-30275.xml | 28.8 KB 28.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_30275.png emd_30275.png | 53.9 KB | ||
| Masks |  emd_30275_msk_1.map emd_30275_msk_1.map | 125 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-30275.cif.gz emd-30275.cif.gz | 8.2 KB | ||
| Others |  emd_30275_half_map_1.map.gz emd_30275_half_map_1.map.gz emd_30275_half_map_2.map.gz emd_30275_half_map_2.map.gz | 116 MB 116 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-30275 http://ftp.pdbj.org/pub/emdb/structures/EMD-30275 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-30275 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-30275 | HTTPS FTP |
-Validation report
| Summary document |  emd_30275_validation.pdf.gz emd_30275_validation.pdf.gz | 1 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_30275_full_validation.pdf.gz emd_30275_full_validation.pdf.gz | 1 MB | Display | |
| Data in XML |  emd_30275_validation.xml.gz emd_30275_validation.xml.gz | 14 KB | Display | |
| Data in CIF |  emd_30275_validation.cif.gz emd_30275_validation.cif.gz | 16.6 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-30275 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-30275 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-30275 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-30275 | HTTPS FTP |
-Related structure data
| Related structure data |  7c2kMC  7bzfC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_30275.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_30275.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.82 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
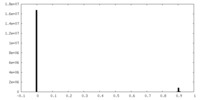
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_30275_msk_1.map emd_30275_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
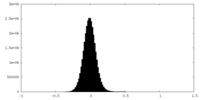
| Density Histograms |
-Half map: #2
| File | emd_30275_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_30275_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : COVID-19 RNA-dependent RNA polymerase pre-translocated catalytic ...
| Entire | Name: COVID-19 RNA-dependent RNA polymerase pre-translocated catalytic complex |
|---|---|
| Components |
|
-Supramolecule #1: COVID-19 RNA-dependent RNA polymerase pre-translocated catalytic ...
| Supramolecule | Name: COVID-19 RNA-dependent RNA polymerase pre-translocated catalytic complex type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#5 Details: full-length COVID-19 nsp12 (residues S1-Q932) was incubated with nsp7 (residues S1-Q83) and nsp8 (A1-Q198), and in complex with RNA template and product. |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: RNA-directed RNA polymerase
| Macromolecule | Name: RNA-directed RNA polymerase / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO / EC number: RNA-directed RNA polymerase |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 108.350703 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGSADAQSFL NRVCGVSAAR LTPCGTGTST DVVYRAFDIY NDKVAGFAKF LKTNCCRFQE KDEDDNLIDS YFVVKRHTFS NYQHEETIY NLLKDCPAVA KHDFFKFRID GDMVPHISRQ RLTKYTMADL VYALRHFDEG NCDTLKEILV TYNCCDDDYF N KKDWYDFV ...String: MGSADAQSFL NRVCGVSAAR LTPCGTGTST DVVYRAFDIY NDKVAGFAKF LKTNCCRFQE KDEDDNLIDS YFVVKRHTFS NYQHEETIY NLLKDCPAVA KHDFFKFRID GDMVPHISRQ RLTKYTMADL VYALRHFDEG NCDTLKEILV TYNCCDDDYF N KKDWYDFV ENPDILRVYA NLGERVRQAL LKTVQFCDAM RNAGIVGVLT LDNQDLNGNW YDFGDFIQTT PGSGVPVVDS YY SLLMPIL TLTRALTAES HVDTDLTKPY IKWDLLKYDF TEERLKLFDR YFKYWDQTYH PNCVNCLDDR CILHCANFNV LFS TVFPPT SFGPLVRKIF VDGVPFVVST GYHFRELGVV HNQDVNLHSS RLSFKELLVY AADPAMHAAS GNLLLDKRTT CFSV AALTN NVAFQTVKPG NFNKDFYDFA VSKGFFKEGS SVELKHFFFA QDGNAAISDY DYYRYNLPTM CDIRQLLFVV EVVDK YFDC YDGGCINANQ VIVNNLDKSA GFPFNKWGKA RLYYDSMSYE DQDALFAYTK RNVIPTITQM NLKYAISAKN RARTVA GVS ICSTMTNRQF HQKLLKSIAA TRGATVVIGT SKFYGGWHNM LKTVYSDVEN PHLMGWDYPK CDRAMPNMLR IMASLVL AR KHTTCCSLSH RFYRLANECA QVLSEMVMCG GSLYVKPGGT SSGDATTAYA NSVFNICQAV TANVNALLST DGNKIADK Y VRNLQHRLYE CLYRNRDVDT DFVNEFYAYL RKHFSMMILS DDAVVCFNST YASQGLVASI KNFKSVLYYQ NNVFMSEAK CWTETDLTKG PHEFCSQHTM LVKQGDDYVY LPYPDPSRIL GAGCFVDDIV KTDGTLMIER FVSLAIDAYP LTKHPNQEYA DVFHLYLQY IRKLHDELTG HMLDMYSVML TNDNTSRYWE PEFYEAMYTP HTVLQHHHHH HHHHH UniProtKB: Replicase polyprotein 1ab |
-Macromolecule #2: Non-structural protein 8
| Macromolecule | Name: Non-structural protein 8 / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 22.057213 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GPAIASEFSS LPSYAAFATA QEAYEQAVAN GDSEVVLKKL KKSLNVAKSE FDRDAAMQRK LEKMADQAMT QMYKQARSED KRAKVTSAM QTMLFTMLRK LDNDALNNII NNARDGCVPL NIIPLTTAAK LMVVIPDYNT YKNTCDGTTF TYASALWEIQ Q VVDADSKI ...String: GPAIASEFSS LPSYAAFATA QEAYEQAVAN GDSEVVLKKL KKSLNVAKSE FDRDAAMQRK LEKMADQAMT QMYKQARSED KRAKVTSAM QTMLFTMLRK LDNDALNNII NNARDGCVPL NIIPLTTAAK LMVVIPDYNT YKNTCDGTTF TYASALWEIQ Q VVDADSKI VQLSEISMDN SPNLAWPLIV TALRANSAVK LQ UniProtKB: Replicase polyprotein 1ab |
-Macromolecule #3: Non-structural protein 7
| Macromolecule | Name: Non-structural protein 7 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 9.402971 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GPSKMSDVKC TSVVLLSVLQ QLRVESSSKL WAQCVQLHND ILLAKDTTEA FEKMVSLLSV LLSMQGAVDI NKLCEEMLDN RATLQ UniProtKB: Replicase polyprotein 1ab |
-Macromolecule #4: RNA (29-MER)
| Macromolecule | Name: RNA (29-MER) / type: rna / ID: 4 / Number of copies: 1 |
|---|---|
| Source (natural) | Organism:   Foot-and-mouth disease virus Foot-and-mouth disease virus |
| Molecular weight | Theoretical: 9.293521 KDa |
| Sequence | String: GGGAGAUGUC UCCUCCUGUG UCGUCGAAA |
-Macromolecule #5: RNA (5'-R(*UP*GP*UP*UP*CP*GP*AP*CP*GP*AP*CP*AP*CP*AP*GP*G*(F86)P*...
| Macromolecule | Name: RNA (5'-R(*UP*GP*UP*UP*CP*GP*AP*CP*GP*AP*CP*AP*CP*AP*GP*G*(F86)P*G)-3') type: rna / ID: 5 / Number of copies: 1 |
|---|---|
| Source (natural) | Organism:   Foot-and-mouth disease virus Foot-and-mouth disease virus |
| Molecular weight | Theoretical: 5.835554 KDa |
| Sequence | String: UGUUCGACGA CACAGG(F86)G |
-Macromolecule #6: ZINC ION
| Macromolecule | Name: ZINC ION / type: ligand / ID: 6 / Number of copies: 2 / Formula: ZN |
|---|---|
| Molecular weight | Theoretical: 65.409 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 5 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | #0 - Image recording ID: 1 / #0 - Film or detector model: GATAN K2 QUANTUM (4k x 4k) / #0 - Detector mode: SUPER-RESOLUTION / #0 - Average electron dose: 60.0 e/Å2 / #1 - Image recording ID: 2 / #1 - Film or detector model: GATAN K2 QUANTUM (4k x 4k) / #1 - Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)