[English] 日本語
 Yorodumi
Yorodumi- EMDB-29929: Domoate-bound GluK2 kainate receptors in non-active conformation -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Domoate-bound GluK2 kainate receptors in non-active conformation | |||||||||
 Map data Map data | Domoate-bound GluK2 kainate receptors in non-active conformation | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | ion channel / glutamate kainate receptor 2 / homotetramer / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationmossy fiber rosette / detection of cold stimulus involved in thermoception / Activation of Na-permeable kainate receptors / kainate selective glutamate receptor complex / Activation of Ca-permeable Kainate Receptor / regulation of short-term neuronal synaptic plasticity / glutamate receptor activity / ubiquitin conjugating enzyme binding / negative regulation of synaptic transmission, glutamatergic / regulation of JNK cascade ...mossy fiber rosette / detection of cold stimulus involved in thermoception / Activation of Na-permeable kainate receptors / kainate selective glutamate receptor complex / Activation of Ca-permeable Kainate Receptor / regulation of short-term neuronal synaptic plasticity / glutamate receptor activity / ubiquitin conjugating enzyme binding / negative regulation of synaptic transmission, glutamatergic / regulation of JNK cascade / inhibitory postsynaptic potential / receptor clustering / kainate selective glutamate receptor activity / modulation of excitatory postsynaptic potential / extracellularly glutamate-gated ion channel activity / ionotropic glutamate receptor complex / positive regulation of synaptic transmission / behavioral fear response / neuronal action potential / glutamate-gated receptor activity / glutamate-gated calcium ion channel activity / ligand-gated monoatomic ion channel activity involved in regulation of presynaptic membrane potential / presynaptic modulation of chemical synaptic transmission / dendrite cytoplasm / hippocampal mossy fiber to CA3 synapse / SNARE binding / regulation of membrane potential / PDZ domain binding / transmitter-gated monoatomic ion channel activity involved in regulation of postsynaptic membrane potential / synaptic transmission, glutamatergic / excitatory postsynaptic potential / regulation of long-term neuronal synaptic plasticity / postsynaptic density membrane / modulation of chemical synaptic transmission / terminal bouton / intracellular calcium ion homeostasis / positive regulation of neuron apoptotic process / presynaptic membrane / neuron apoptotic process / scaffold protein binding / perikaryon / chemical synaptic transmission / negative regulation of neuron apoptotic process / postsynaptic membrane / postsynaptic density / axon / neuronal cell body / ubiquitin protein ligase binding / synapse / dendrite / glutamatergic synapse / identical protein binding / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |  | |||||||||
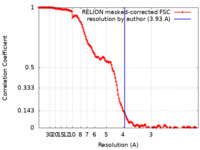
| Method | single particle reconstruction / cryo EM / Resolution: 3.93 Å | |||||||||
 Authors Authors | Bogdanovic N / Tajima N | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2025 Journal: Nat Struct Mol Biol / Year: 2025Title: Structural basis of GluK2 kainate receptor activation by a partial agonist. Authors: Guadalupe Segura-Covarrubias / Changping Zhou / Nebojša Bogdanović / Lisa Zhang / Nami Tajima /  Abstract: Kainate receptors (KARs) belong to the family of ionotropic glutamate receptors that regulate neurotransmitter release and excitatory synaptic transmission in the central nervous system. Despite ...Kainate receptors (KARs) belong to the family of ionotropic glutamate receptors that regulate neurotransmitter release and excitatory synaptic transmission in the central nervous system. Despite their critical roles in synaptic signaling and disease, the detailed gating mechanisms of KARs are not completely understood. Here we present cryo-electron microscopy structures of homomeric rat GluK2 KAR in an unliganded apo state and in complexes with a partial agonist, domoate. Partial agonist-bound GluK2 populates multiple conformations, including intermediate and desensitized states. Moreover, we demonstrate that the N-glycans at the amino-terminal domain-ligand binding domain (LBD) interface modulate receptor gating properties by interfering with cation binding at the LBD dimer interface. Together, these results provide insights into the unique gating mechanisms of KARs. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_29929.map.gz emd_29929.map.gz | 226.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-29929-v30.xml emd-29929-v30.xml emd-29929.xml emd-29929.xml | 22.4 KB 22.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_29929_fsc.xml emd_29929_fsc.xml | 14.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_29929.png emd_29929.png | 74.3 KB | ||
| Masks |  emd_29929_msk_1.map emd_29929_msk_1.map | 282.6 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-29929.cif.gz emd-29929.cif.gz | 7.3 KB | ||
| Others |  emd_29929_half_map_1.map.gz emd_29929_half_map_1.map.gz emd_29929_half_map_2.map.gz emd_29929_half_map_2.map.gz | 226.4 MB 225.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-29929 http://ftp.pdbj.org/pub/emdb/structures/EMD-29929 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29929 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29929 | HTTPS FTP |
-Related structure data
| Related structure data |  8gc5MC  9c5yC  9c5zC  9c60C  9cazC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_29929.map.gz / Format: CCP4 / Size: 282.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_29929.map.gz / Format: CCP4 / Size: 282.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Domoate-bound GluK2 kainate receptors in non-active conformation | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.06 Å | ||||||||||||||||||||||||||||||||||||
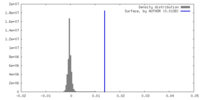
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_29929_msk_1.map emd_29929_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half Map 1
| File | emd_29929_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half Map 1 | ||||||||||||
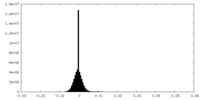
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half Map 2
| File | emd_29929_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half Map 2 | ||||||||||||
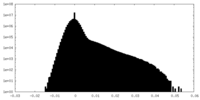
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Homotetrameric GluK2 bound with DOQ state 4
| Entire | Name: Homotetrameric GluK2 bound with DOQ state 4 |
|---|---|
| Components |
|
-Supramolecule #1: Homotetrameric GluK2 bound with DOQ state 4
| Supramolecule | Name: Homotetrameric GluK2 bound with DOQ state 4 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 Details: Tetramer isolated and dyalised extensively. The DOQ was applied prior to grid freezing. |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Glutamate receptor ionotropic, kainate 2
| Macromolecule | Name: Glutamate receptor ionotropic, kainate 2 / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 98.883469 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: HVLRFGGIFE YVESGPMGAE ELAFRFAVNT INRNRTLLPN TTLTYDTQKI NLYDSFEASK KACDQLSLGV AAIFGPSHSS SANAVQSIC NALGVPHIQT RWKHQVSDNK DSFYVSLYPD FSSLSRAILD LVQFFKWKTV TVVYDDSTGL IRLQELIKAP S RYNLRLKI ...String: HVLRFGGIFE YVESGPMGAE ELAFRFAVNT INRNRTLLPN TTLTYDTQKI NLYDSFEASK KACDQLSLGV AAIFGPSHSS SANAVQSIC NALGVPHIQT RWKHQVSDNK DSFYVSLYPD FSSLSRAILD LVQFFKWKTV TVVYDDSTGL IRLQELIKAP S RYNLRLKI RQLPADTKDA KPLLKEMKRG KEFHVIFDCS HEMAAGILKQ ALAMGMMTEY YHYIFTTLDL FALDVEPYRY SG VNMTGFR ILNTENTQVS SIIEKWSMER LQAPPKPDSG LLDGFMTTDA ALMYDAVHVV SVAVQQFPQM TVSSLQCNRH KPW RFGTRF MSLIKEAHWE GLTGRITFNK TNGLRTDFDL DVISLKEEGL EKIGTWDPAS GLNMTESQKG KPANITDSLS NRSL IVTTI LEEPYVLFKK SDKPLYGNDR FEGYCIDLLR ELSTILGFTY EIRLVEDGKY GAQDDVNGQW NGMVRELIDH KADLA VAPL AITYVREKVI DFSKPFMTLG ISILYRKPNG TNPGVFSFLN PLSPDIWMYV LLAYLGVSVV LFVIARFSPY EWYNPH PSN PDSDVVENNF TLLNSFWFGV GALMQQGSEL MPKALSTRIV GGIWWFFTLI IISSYTANLA AFLTVERMES PIDSADD LA KQTKIEYGAV EDGATMTFFK KSKISTYDKM WAFMSSRRQS VLVKSNEEGI QRVLTSDYAF LMESTTIEFV TQRNCNLT Q IGGLIDSKGY GVGTPMGSPY RDKITIAILQ LQEEGKLHMM KEKWWRGNGC PEEESKEASA LGVQNIGGIF IVLAAGLVL SVFVAVGEFL YKSKKNAQLE KRSFCSAMVE ELRMSLKCQR RLKHKPQAPV IVKTEEVINM HTFNDRRLPG KETMA UniProtKB: Glutamate receptor ionotropic, kainate 2 |
-Macromolecule #5: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 5 / Number of copies: 6 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Macromolecule #6: (2S,3S,4S)-2-CARBOXY-4-[(1Z,3E,5R)-5-CARBOXY-1-METHYL-1,3-HEXADIE...
| Macromolecule | Name: (2S,3S,4S)-2-CARBOXY-4-[(1Z,3E,5R)-5-CARBOXY-1-METHYL-1,3-HEXADIENYL]-3-PYRROLIDINEACETIC ACID type: ligand / ID: 6 / Number of copies: 4 / Formula: DOQ |
|---|---|
| Molecular weight | Theoretical: 311.33 Da |
| Chemical component information |  ChemComp-DOQ: |
-Macromolecule #7: alpha-D-mannopyranose
| Macromolecule | Name: alpha-D-mannopyranose / type: ligand / ID: 7 / Number of copies: 1 / Formula: MAN |
|---|---|
| Molecular weight | Theoretical: 180.156 Da |
| Chemical component information |  ChemComp-MAN: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 3.5 mg/mL |
|---|---|
| Buffer | pH: 8 / Component - Concentration: 150.0 mmol / Component - Formula: NaCl / Component - Name: sodium chloride / Details: 50 mM Tris/HCl 150 mM NaCl 1mM DDM pH 8.0 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277.15 K / Instrument: FEI VITROBOT MARK IV |
| Details | The sample was isolated from SEC and concentrated. Monodispersity of the final sample determined by the analytical HPLC, Superose 6 increase column |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Number grids imaged: 1 / Number real images: 12486 / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: OTHER / Cs: 2.7 mm / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 81000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: RIGID BODY FIT / Overall B value: 70 / Target criteria: 0.85 |
|---|---|
| Output model |  PDB-8gc5: |
 Movie
Movie Controller
Controller









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)