+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM structure of Go-coupled NTSR1 | |||||||||
 Map data Map data | CryoEM structure of Go-coupled NTSR1 | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | GPCR / neurotensin receptor / allosterism / SBI-553 / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of locomotion involved in locomotory behavior / Peptide ligand-binding receptors / positive regulation of locomotion / G protein-coupled neurotensin receptor activity / neuropeptide receptor binding / regulation of inositol trisphosphate biosynthetic process / inositol phosphate catabolic process / symmetric synapse / D-aspartate import across plasma membrane / positive regulation of gamma-aminobutyric acid secretion ...regulation of locomotion involved in locomotory behavior / Peptide ligand-binding receptors / positive regulation of locomotion / G protein-coupled neurotensin receptor activity / neuropeptide receptor binding / regulation of inositol trisphosphate biosynthetic process / inositol phosphate catabolic process / symmetric synapse / D-aspartate import across plasma membrane / positive regulation of gamma-aminobutyric acid secretion / regulation of membrane depolarization / positive regulation of arachidonate secretion / vocalization behavior / response to antipsychotic drug / neuron spine / L-glutamate import across plasma membrane / regulation of behavioral fear response / regulation of respiratory gaseous exchange / neuropeptide hormone activity / cAMP biosynthetic process / mu-type opioid receptor binding / corticotropin-releasing hormone receptor 1 binding / positive regulation of inhibitory postsynaptic potential / negative regulation of systemic arterial blood pressure / negative regulation of release of sequestered calcium ion into cytosol / vesicle docking involved in exocytosis / positive regulation of glutamate secretion / G alpha (q) signalling events / digestive tract development / hyperosmotic response / response to mineralocorticoid / response to food / response to corticosterone / cellular response to lithium ion / G protein-coupled dopamine receptor signaling pathway / temperature homeostasis / response to lipid / positive regulation of inositol phosphate biosynthetic process / regulation of heart contraction / detection of temperature stimulus involved in sensory perception of pain / parallel fiber to Purkinje cell synapse / response to stress / associative learning / conditioned place preference / cellular response to dexamethasone stimulus / neuropeptide signaling pathway / response to axon injury / postsynaptic modulation of chemical synaptic transmission / transport vesicle / axon terminus / G protein-coupled serotonin receptor binding / adenylate cyclase regulator activity / adenylate cyclase-inhibiting serotonin receptor signaling pathway / muscle contraction / positive regulation of release of sequestered calcium ion into cytosol / response to amphetamine / blood vessel diameter maintenance / dendritic shaft / adult locomotory behavior / learning / response to cocaine / locomotory behavior / liver development / cellular response to nerve growth factor stimulus / negative regulation of insulin secretion / visual learning / GABA-ergic synapse / cytoplasmic side of plasma membrane / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / G-protein beta/gamma-subunit complex binding / Olfactory Signaling Pathway / terminal bouton / Activation of the phototransduction cascade / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / G protein-coupled acetylcholine receptor signaling pathway / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / G-protein activation / G beta:gamma signalling through CDC42 / Prostacyclin signalling through prostacyclin receptor / Glucagon signaling in metabolic regulation / G beta:gamma signalling through BTK / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / ADP signalling through P2Y purinoceptor 12 / photoreceptor disc membrane / Glucagon-type ligand receptors / Sensory perception of sweet, bitter, and umami (glutamate) taste / Adrenaline,noradrenaline inhibits insulin secretion / Vasopressin regulates renal water homeostasis via Aquaporins / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / G alpha (z) signalling events / ADP signalling through P2Y purinoceptor 1 / cellular response to catecholamine stimulus / ADORA2B mediated anti-inflammatory cytokines production / G beta:gamma signalling through PI3Kgamma / response to estradiol / adenylate cyclase-activating dopamine receptor signaling pathway / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding Similarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) / Homo sapiens (human) /  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.88 Å | |||||||||
 Authors Authors | Krumm BE / DiBerto JF / Olsen RHJ / Kang H / Slocum ST / Zhang S / Strachan RT / Fay JF / Roth BL | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Biochemistry / Year: 2023 Journal: Biochemistry / Year: 2023Title: Neurotensin Receptor Allosterism Revealed in Complex with a Biased Allosteric Modulator. Authors: Brian E Krumm / Jeffrey F DiBerto / Reid H J Olsen / Hye Jin Kang / Samuel T Slocum / Shicheng Zhang / Ryan T Strachan / Xi-Ping Huang / Lauren M Slosky / Anthony B Pinkerton / Lawrence S ...Authors: Brian E Krumm / Jeffrey F DiBerto / Reid H J Olsen / Hye Jin Kang / Samuel T Slocum / Shicheng Zhang / Ryan T Strachan / Xi-Ping Huang / Lauren M Slosky / Anthony B Pinkerton / Lawrence S Barak / Marc G Caron / Terry Kenakin / Jonathan F Fay / Bryan L Roth /  Abstract: The NTSR1 neurotensin receptor (NTSR1) is a G protein-coupled receptor (GPCR) found in the brain and peripheral tissues with neurotensin (NTS) being its endogenous peptide ligand. In the brain, NTS ...The NTSR1 neurotensin receptor (NTSR1) is a G protein-coupled receptor (GPCR) found in the brain and peripheral tissues with neurotensin (NTS) being its endogenous peptide ligand. In the brain, NTS modulates dopamine neuronal activity, induces opioid-independent analgesia, and regulates food intake. Recent studies indicate that biasing NTSR1 toward β-arrestin signaling can attenuate the actions of psychostimulants and other drugs of abuse. Here, we provide the cryoEM structures of NTSR1 ternary complexes with heterotrimeric Gq and GoA with and without the brain-penetrant small-molecule SBI-553. In functional studies, we discovered that SBI-553 displays complex allosteric actions exemplified by negative allosteric modulation for G proteins that are Gα subunit selective and positive allosteric modulation and agonism for β-arrestin translocation at NTSR1. Detailed structural analysis of the allosteric binding site illuminated the structural determinants for biased allosteric modulation of SBI-553 on NTSR1. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_29303.map.gz emd_29303.map.gz | 55 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-29303-v30.xml emd-29303-v30.xml emd-29303.xml emd-29303.xml | 20.9 KB 20.9 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_29303.png emd_29303.png | 24.7 KB | ||
| Filedesc metadata |  emd-29303.cif.gz emd-29303.cif.gz | 6.8 KB | ||
| Others |  emd_29303_half_map_1.map.gz emd_29303_half_map_1.map.gz emd_29303_half_map_2.map.gz emd_29303_half_map_2.map.gz | 59.4 MB 59.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-29303 http://ftp.pdbj.org/pub/emdb/structures/EMD-29303 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29303 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29303 | HTTPS FTP |
-Related structure data
| Related structure data |  8fn1MC  8fmzC  8fn0C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_29303.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_29303.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | CryoEM structure of Go-coupled NTSR1 | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.88 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Half Map 1
| File | emd_29303_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half Map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
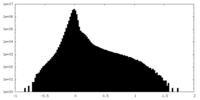
| Density Histograms |
-Half map: Half Map 2
| File | emd_29303_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half Map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
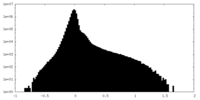
| Density Histograms |
- Sample components
Sample components
-Entire : Ternary complex of NTSR1 MiniGo heterotrimer with scFv16
| Entire | Name: Ternary complex of NTSR1 MiniGo heterotrimer with scFv16 |
|---|---|
| Components |
|
-Supramolecule #1: Ternary complex of NTSR1 MiniGo heterotrimer with scFv16
| Supramolecule | Name: Ternary complex of NTSR1 MiniGo heterotrimer with scFv16 type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 147 KDa |
-Macromolecule #1: Neurotensin/neuromedin N
| Macromolecule | Name: Neurotensin/neuromedin N / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 819.007 Da |
| Sequence | String: RRPYIL UniProtKB: Neurotensin/neuromedin N |
-Macromolecule #2: Neurotensin receptor type 1
| Macromolecule | Name: Neurotensin receptor type 1 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 45.842098 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MHHHHHHHHH HSDLEVLFQG PLGSGATSES DTAGPNSDLD VNTDIYSKVL VTAIYLALFV VGTVGNSVTL FTLARKKSLQ SLQSTVHYH LGSLALSDLL ILLLAMPVEL YNFIWVHHPW AFGDAGCRGY YFLRDACTYA TALNVASLSV ERYLAICHPF K AKTLMSRS ...String: MHHHHHHHHH HSDLEVLFQG PLGSGATSES DTAGPNSDLD VNTDIYSKVL VTAIYLALFV VGTVGNSVTL FTLARKKSLQ SLQSTVHYH LGSLALSDLL ILLLAMPVEL YNFIWVHHPW AFGDAGCRGY YFLRDACTYA TALNVASLSV ERYLAICHPF K AKTLMSRS RTKKFISAIW LASALLAIPM LFTMGLQNRS ADGTHPGGLV CTPIVDTATV KVVIQVNTFM SFLFPMLVIS IL NTVIANK LTVMVHQAAE QGRVCTVGTH NGLEHSTFNM TIEPGRVQAL RHGVLVLRAV VIAFVVCWLP YHVRRLMFCY ISD EQWTTF LFDFYHYFYM LTNALFYASS AINPILYNLV SANFRQVFLS TLACLCPGWR HRRKKRPTFS RKPNSMSSNH AFST SATRE TLY UniProtKB: Neurotensin receptor type 1 |
-Macromolecule #3: Guanine nucleotide-binding protein G(o) subunit alpha
| Macromolecule | Name: Guanine nucleotide-binding protein G(o) subunit alpha / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 25.451166 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGCTLSAEDK AAVERSKMIE KNLKEDGISA AKDVKLLLLG ADNSGKSTIV KQMKIIHGGS GGSGGTTGIV ETHFTFKNLH FRLFDVGGQ RSERKKWIHC FEDVTAIIFC VDLSDYNRMH ESLMLFDSIC NNKFFIDTSI ILFLNKKDLF GEKIKKSPLT I CFPEYTGP ...String: MGCTLSAEDK AAVERSKMIE KNLKEDGISA AKDVKLLLLG ADNSGKSTIV KQMKIIHGGS GGSGGTTGIV ETHFTFKNLH FRLFDVGGQ RSERKKWIHC FEDVTAIIFC VDLSDYNRMH ESLMLFDSIC NNKFFIDTSI ILFLNKKDLF GEKIKKSPLT I CFPEYTGP NTYEDAAAYI QAQFESKNRS PNKEIYCHMT CATDTNNAQV IFDAVTDIII ANNLRGCGLY UniProtKB: Guanine nucleotide-binding protein G(o) subunit alpha, Guanine nucleotide-binding protein G(o) subunit alpha |
-Macromolecule #4: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 39.418086 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MHHHHHHLEV LFQGPGSSGS ELDQLRQEAE QLKNQIRDAR KACADATLSQ ITNNIDPVGR IQMRTRRTLR GHLAKIYAMH WGTDSRLLV SASQDGKLII WDSYTTNKVH AIPLRSSWVM TCAYAPSGNY VACGGLDNIC SIYNLKTREG NVRVSRELAG H TGYLSCCR ...String: MHHHHHHLEV LFQGPGSSGS ELDQLRQEAE QLKNQIRDAR KACADATLSQ ITNNIDPVGR IQMRTRRTLR GHLAKIYAMH WGTDSRLLV SASQDGKLII WDSYTTNKVH AIPLRSSWVM TCAYAPSGNY VACGGLDNIC SIYNLKTREG NVRVSRELAG H TGYLSCCR FLDDNQIVTS SGDTTCALWD IETGQQTTTF TGHTGDVMSL SLAPDTRLFV SGACDASAKL WDVREGMCRQ TF TGHESDI NAICFFPNGN AFATGSDDAT CRLFDLRADQ ELMTYSHDNI ICGITSVSFS KSGRLLLAGY DDFNCNVWDA LKA DRAGVL AGHDNRVSCL GVTDDGMAVA TGSWDSFLKI WN UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 |
-Macromolecule #5: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 7.861143 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MASNNTASIA QARKLVEQLK MEANIDRIKV SKAAADLMAY CEAHAKEDPL LTPVPASENP FREKKFFCAI L UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 |
-Macromolecule #6: scFv16
| Macromolecule | Name: scFv16 / type: protein_or_peptide / ID: 6 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 28.668922 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: DVQLVESGGG LVQPGGSRKL SCSASGFAFS SFGMHWVRQA PEKGLEWVAY ISSGSGTIYY ADTVKGRFTI SRDDPKNTLF LQMTSLRSE DTAMYYCVRS IYYYGSSPFD FWGQGTTLTV SSGGGGSGGG GSGGGGSDIV MTQATSSVPV TPGESVSISC R SSKSLLHS ...String: DVQLVESGGG LVQPGGSRKL SCSASGFAFS SFGMHWVRQA PEKGLEWVAY ISSGSGTIYY ADTVKGRFTI SRDDPKNTLF LQMTSLRSE DTAMYYCVRS IYYYGSSPFD FWGQGTTLTV SSGGGGSGGG GSGGGGSDIV MTQATSSVPV TPGESVSISC R SSKSLLHS NGNTYLYWFL QRPGQSPQLL IYRMSNLASG VPDRFSGSGS GTAFTLTISR LEAEDVGVYY CMQHLEYPLT FG AGTKLEL KAAALEVLFQ GPHHHHHHHH |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 / Details: 20mM Hepes, 0.1M NaCl, 0.00075% LMNG, 0.00075% GDN |
|---|---|
| Grid | Model: Quantifoil / Material: GOLD / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE-PROPANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TALOS ARCTICA |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 44.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Nominal defocus max: 3.2 µm / Nominal defocus min: 0.1 µm |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: PDB ENTRY PDB model - PDB ID: |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 2.88 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 560249 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller




























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)