+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | momSalB bound Kappa Opioid Receptor in complex with Gi1 | |||||||||
 Map data Map data | deep sharp | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | GPCR / KAPPA OPIOID Receptor / SALVINORIN / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationresponse to acrylamide / adenylate cyclase-inhibiting opioid receptor signaling pathway / dynorphin receptor activity / regulation of saliva secretion / negative regulation of luteinizing hormone secretion / sensory perception of temperature stimulus / positive regulation of eating behavior / G protein-coupled opioid receptor activity / G protein-coupled opioid receptor signaling pathway / positive regulation of dopamine secretion ...response to acrylamide / adenylate cyclase-inhibiting opioid receptor signaling pathway / dynorphin receptor activity / regulation of saliva secretion / negative regulation of luteinizing hormone secretion / sensory perception of temperature stimulus / positive regulation of eating behavior / G protein-coupled opioid receptor activity / G protein-coupled opioid receptor signaling pathway / positive regulation of dopamine secretion / sensory perception / maternal behavior / positive regulation of potassium ion transmembrane transport / receptor serine/threonine kinase binding / positive regulation of p38MAPK cascade / neuropeptide binding / eating behavior / conditioned place preference / neuropeptide signaling pathway / estrous cycle / adenylate cyclase inhibitor activity / MECP2 regulates neuronal receptors and channels / positive regulation of protein localization to cell cortex / behavioral response to cocaine / T cell migration / Adenylate cyclase inhibitory pathway / D2 dopamine receptor binding / response to prostaglandin E / axon terminus / adenylate cyclase regulator activity / G protein-coupled serotonin receptor binding / adenylate cyclase-inhibiting serotonin receptor signaling pathway / sensory perception of pain / T-tubule / cellular response to forskolin / Peptide ligand-binding receptors / regulation of mitotic spindle organization / sarcoplasmic reticulum / response to nicotine / Regulation of insulin secretion / locomotory behavior / cellular response to glucose stimulus / positive regulation of cholesterol biosynthetic process / negative regulation of insulin secretion / G protein-coupled receptor binding / response to insulin / response to peptide hormone / adenylate cyclase-inhibiting G protein-coupled receptor signaling pathway / response to estrogen / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / centriolar satellite / G-protein beta/gamma-subunit complex binding / Olfactory Signaling Pathway / Activation of the phototransduction cascade / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / G protein-coupled acetylcholine receptor signaling pathway / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / G-protein activation / G beta:gamma signalling through CDC42 / Prostacyclin signalling through prostacyclin receptor / Glucagon signaling in metabolic regulation / G beta:gamma signalling through BTK / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / ADP signalling through P2Y purinoceptor 12 / photoreceptor disc membrane / Sensory perception of sweet, bitter, and umami (glutamate) taste / Glucagon-type ligand receptors / Adrenaline,noradrenaline inhibits insulin secretion / synaptic vesicle membrane / GDP binding / Vasopressin regulates renal water homeostasis via Aquaporins / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / G alpha (z) signalling events / ADP signalling through P2Y purinoceptor 1 / cellular response to catecholamine stimulus / ADORA2B mediated anti-inflammatory cytokines production / G beta:gamma signalling through PI3Kgamma / adenylate cyclase-activating dopamine receptor signaling pathway / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / GPER1 signaling / G-protein beta-subunit binding / cellular response to prostaglandin E stimulus / heterotrimeric G-protein complex / Inactivation, recovery and regulation of the phototransduction cascade / G alpha (12/13) signalling events / extracellular vesicle / sensory perception of taste / Thrombin signalling through proteinase activated receptors (PARs) / signaling receptor complex adaptor activity / cellular response to lipopolysaccharide / G protein activity / retina development in camera-type eye / presynaptic membrane / GTPase binding / Ca2+ pathway / fibroblast proliferation / response to ethanol Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.71 Å | |||||||||
 Authors Authors | Fay JF / Che T | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2023 Journal: Nature / Year: 2023Title: Ligand and G-protein selectivity in the κ-opioid receptor. Authors: Jianming Han / Jingying Zhang / Antonina L Nazarova / Sarah M Bernhard / Brian E Krumm / Lei Zhao / Jordy Homing Lam / Vipin A Rangari / Susruta Majumdar / David E Nichols / Vsevolod ...Authors: Jianming Han / Jingying Zhang / Antonina L Nazarova / Sarah M Bernhard / Brian E Krumm / Lei Zhao / Jordy Homing Lam / Vipin A Rangari / Susruta Majumdar / David E Nichols / Vsevolod Katritch / Peng Yuan / Jonathan F Fay / Tao Che /  Abstract: The κ-opioid receptor (KOR) represents a highly desirable therapeutic target for treating not only pain but also addiction and affective disorders. However, the development of KOR analgesics has ...The κ-opioid receptor (KOR) represents a highly desirable therapeutic target for treating not only pain but also addiction and affective disorders. However, the development of KOR analgesics has been hindered by the associated hallucinogenic side effects. The initiation of KOR signalling requires the G-family proteins including the conventional (G, G, G, G and G) and nonconventional (G and G) subtypes. How hallucinogens exert their actions through KOR and how KOR determines G-protein subtype selectivity are not well understood. Here we determined the active-state structures of KOR in a complex with multiple G-protein heterotrimers-G, G, G and G-using cryo-electron microscopy. The KOR-G-protein complexes are bound to hallucinogenic salvinorins or highly selective KOR agonists. Comparisons of these structures reveal molecular determinants critical for KOR-G-protein interactions as well as key elements governing G-family subtype selectivity and KOR ligand selectivity. Furthermore, the four G-protein subtypes display an intrinsically different binding affinity and allosteric activity on agonist binding at KOR. These results provide insights into the actions of opioids and G-protein-coupling specificity at KOR and establish a foundation to examine the therapeutic potential of pathway-selective agonists of KOR. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_27804.map.gz emd_27804.map.gz | 76.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-27804-v30.xml emd-27804-v30.xml emd-27804.xml emd-27804.xml | 26.2 KB 26.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_27804.png emd_27804.png | 37.4 KB | ||
| Filedesc metadata |  emd-27804.cif.gz emd-27804.cif.gz | 7.4 KB | ||
| Others |  emd_27804_additional_1.map.gz emd_27804_additional_1.map.gz emd_27804_half_map_1.map.gz emd_27804_half_map_1.map.gz emd_27804_half_map_2.map.gz emd_27804_half_map_2.map.gz | 85 MB 84.5 MB 84.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-27804 http://ftp.pdbj.org/pub/emdb/structures/EMD-27804 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27804 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27804 | HTTPS FTP |
-Related structure data
| Related structure data |  8dzpMC  8dzqC  8dzrC  8dzsC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_27804.map.gz / Format: CCP4 / Size: 91.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_27804.map.gz / Format: CCP4 / Size: 91.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | deep sharp | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.88 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: half A
| File | emd_27804_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: half B
| File | emd_27804_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half B | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: half A
| File | emd_27804_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : momSalB bound Kappa Opioid Receptor in complex with Gi1
| Entire | Name: momSalB bound Kappa Opioid Receptor in complex with Gi1 |
|---|---|
| Components |
|
-Supramolecule #1: momSalB bound Kappa Opioid Receptor in complex with Gi1
| Supramolecule | Name: momSalB bound Kappa Opioid Receptor in complex with Gi1 type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#5 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Kappa-type opioid receptor
| Macromolecule | Name: Kappa-type opioid receptor / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 34.928555 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: LGSISPAIPV IITAVYSVVF VVGLVGNSLV MFVIIRYTKM KTATNIYIFN LALADALVTT TMPFQSTVYL MNSWPFGDVL CKIVLSIDY YNMFTSIFTL TMMSVDRYIA VCHPVKALDF RTPLKAKIIN ICIWLLSSSV GISAIVLGGT KVREDVDVIE C SLQFPDDD ...String: LGSISPAIPV IITAVYSVVF VVGLVGNSLV MFVIIRYTKM KTATNIYIFN LALADALVTT TMPFQSTVYL MNSWPFGDVL CKIVLSIDY YNMFTSIFTL TMMSVDRYIA VCHPVKALDF RTPLKAKIIN ICIWLLSSSV GISAIVLGGT KVREDVDVIE C SLQFPDDD YSWWDLFMKI CVFIFAFVIP VLIIIVCYTL MILRLKSVRL LSGSREKDRN LRRITRLVLV VVAVFVVCWT PI HIFILVE ALGSTSHSTA ALSSYYFCIA LGYTNSSLNP ILYAFLDENF KRCFRDFCFP LKMRMERQST S UniProtKB: Kappa-type opioid receptor |
-Macromolecule #2: Guanine nucleotide-binding protein G(i) subunit alpha-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(i) subunit alpha-1 type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 40.414047 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGCTLSAEDK AAVERSKMID RNLREDGEKA AREVKLLLLG AGESGKNTIV KQMKIIHEAG YSEEECKQYK AVVYSNTIQS IIAIIRAMG RLKIDFGDSA RADDARQLFV LAGAAEEGFM TAELAGVIKR LWKDSGVQAC FNRSREYQLN DSAAYYLNDL D RIAQPNYI ...String: MGCTLSAEDK AAVERSKMID RNLREDGEKA AREVKLLLLG AGESGKNTIV KQMKIIHEAG YSEEECKQYK AVVYSNTIQS IIAIIRAMG RLKIDFGDSA RADDARQLFV LAGAAEEGFM TAELAGVIKR LWKDSGVQAC FNRSREYQLN DSAAYYLNDL D RIAQPNYI PTQQDVLRTR VKTTGIVETH FTFKDLHFKM FDVGAQRSER KKWIHCFEGV TAIIFCVALS DYDLVLAEDE EM NRMHASM KLFDSICNNK WFTDTSIILF LNKKDLFEEK IKKSPLTICY PEYAGSNTYE EAAAYIQCQF EDLNKRKDTK EIY THFTCS TDTKNVQFVF DAVTDVIIKN NLKDCGLF UniProtKB: Guanine nucleotide-binding protein G(i) subunit alpha-1 |
-Macromolecule #3: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 37.342785 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GSELDQLRQE AEQLKNQIRD ARKACADATL SQITNNIDPV GRIQMRTRRT LRGHLAKIYA MHWGTDSRLL VSASQDGKLI IWDSYTTNK VHAIPLRSSW VMTCAYAPSG NYVACGGLDN ICSIYNLKTR EGNVRVSREL AGHTGYLSCC RFLDDNQIVT S SGDTTCAL ...String: GSELDQLRQE AEQLKNQIRD ARKACADATL SQITNNIDPV GRIQMRTRRT LRGHLAKIYA MHWGTDSRLL VSASQDGKLI IWDSYTTNK VHAIPLRSSW VMTCAYAPSG NYVACGGLDN ICSIYNLKTR EGNVRVSREL AGHTGYLSCC RFLDDNQIVT S SGDTTCAL WDIETGQQTT TFTGHTGDVM SLSLAPDTRL FVSGACDASA KLWDVREGMC RQTFTGHESD INAICFFPNG NA FATGSDD ATCRLFDLRA DQELMTYSHD NIICGITSVS FSKSGRLLLA GYDDFNCNVW DALKADRAGV LAGHDNRVSC LGV TDDGMA VATGSWDSFL KIWN UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 |
-Macromolecule #4: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 7.861143 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MASNNTASIA QARKLVEQLK MEANIDRIKV SKAAADLMAY CEAHAKEDPL LTPVPASENP FREKKFFCAI L UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 |
-Macromolecule #5: ScFv16 protein
| Macromolecule | Name: ScFv16 protein / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 26.679721 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: DVQLVESGGG LVQPGGSRKL SCSASGFAFS SFGMHWVRQA PEKGLEWVAY ISSGSGTIYY ADTVKGRFTI SRDDPKNTLF LQMTSLRSE DTAMYYCVRS IYYYGSSPFD FWGQGTTLTV SSGGGGSGGG GSGGGGSDIV MTQATSSVPV TPGESVSISC R SSKSLLHS ...String: DVQLVESGGG LVQPGGSRKL SCSASGFAFS SFGMHWVRQA PEKGLEWVAY ISSGSGTIYY ADTVKGRFTI SRDDPKNTLF LQMTSLRSE DTAMYYCVRS IYYYGSSPFD FWGQGTTLTV SSGGGGSGGG GSGGGGSDIV MTQATSSVPV TPGESVSISC R SSKSLLHS NGNTYLYWFL QRPGQSPQLL IYRMSNLASG VPDRFSGSGS GTAFTLTISR LEAEDVGVYY CMQHLEYPLT FG AGTKLEL KAAA |
-Macromolecule #6: methyl (2S,4aR,6aR,7R,9S,10aS,10bR)-2-(furan-3-yl)-9-(methoxymeth...
| Macromolecule | Name: methyl (2S,4aR,6aR,7R,9S,10aS,10bR)-2-(furan-3-yl)-9-(methoxymethoxy)-6a,10b-dimethyl-4,10-dioxododecahydro-2H-naphtho[2,1-c]pyran-7-carboxylate type: ligand / ID: 6 / Number of copies: 1 / Formula: U99 |
|---|---|
| Molecular weight | Theoretical: 434.48 Da |
| Chemical component information | 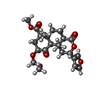 ChemComp-U99: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE-PROPANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TALOS ARCTICA |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number real images: 4175 / Average electron dose: 47.4 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.2 µm / Nominal defocus min: 0.4 µm |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller










































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)