+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | GR89,696 bound Kappa Opioid Receptor in complex with Gz | |||||||||
 Map data Map data | deep sharp | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | GPCR / Kappa opioid receptor / G protein / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationresponse to acrylamide / adenylate cyclase-inhibiting opioid receptor signaling pathway / dynorphin receptor activity / regulation of saliva secretion / negative regulation of luteinizing hormone secretion / sensory perception of temperature stimulus / positive regulation of eating behavior / G protein-coupled serotonin receptor signaling pathway / G protein-coupled opioid receptor activity / G protein-coupled opioid receptor signaling pathway ...response to acrylamide / adenylate cyclase-inhibiting opioid receptor signaling pathway / dynorphin receptor activity / regulation of saliva secretion / negative regulation of luteinizing hormone secretion / sensory perception of temperature stimulus / positive regulation of eating behavior / G protein-coupled serotonin receptor signaling pathway / G protein-coupled opioid receptor activity / G protein-coupled opioid receptor signaling pathway / positive regulation of dopamine secretion / sensory perception / positive regulation of potassium ion transmembrane transport / receptor serine/threonine kinase binding / maternal behavior / positive regulation of p38MAPK cascade / neuropeptide binding / eating behavior / conditioned place preference / neuropeptide signaling pathway / estrous cycle / adenylate cyclase inhibitor activity / MECP2 regulates neuronal receptors and channels / behavioral response to cocaine / T-tubule / G protein-coupled serotonin receptor binding / sensory perception of pain / axon terminus / Peptide ligand-binding receptors / sarcoplasmic reticulum / response to nicotine / locomotory behavior / cellular response to glucose stimulus / negative regulation of insulin secretion / G protein-coupled receptor binding / response to insulin / adenylate cyclase-inhibiting G protein-coupled receptor signaling pathway / response to estrogen / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / G-protein beta/gamma-subunit complex binding / Olfactory Signaling Pathway / Activation of the phototransduction cascade / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / G protein-coupled acetylcholine receptor signaling pathway / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / G-protein activation / G beta:gamma signalling through CDC42 / Prostacyclin signalling through prostacyclin receptor / Glucagon signaling in metabolic regulation / G beta:gamma signalling through BTK / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / ADP signalling through P2Y purinoceptor 12 / photoreceptor disc membrane / Glucagon-type ligand receptors / Sensory perception of sweet, bitter, and umami (glutamate) taste / Adrenaline,noradrenaline inhibits insulin secretion / Vasopressin regulates renal water homeostasis via Aquaporins / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / G alpha (z) signalling events / ADP signalling through P2Y purinoceptor 1 / ADORA2B mediated anti-inflammatory cytokines production / cellular response to catecholamine stimulus / G beta:gamma signalling through PI3Kgamma / nuclear envelope / adenylate cyclase-activating dopamine receptor signaling pathway / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / GPER1 signaling / synaptic vesicle membrane / G-protein beta-subunit binding / cellular response to prostaglandin E stimulus / heterotrimeric G-protein complex / G alpha (12/13) signalling events / Inactivation, recovery and regulation of the phototransduction cascade / extracellular vesicle / sensory perception of taste / Thrombin signalling through proteinase activated receptors (PARs) / signaling receptor complex adaptor activity / cell body / retina development in camera-type eye / cellular response to lipopolysaccharide / GTPase binding / presynaptic membrane / Ca2+ pathway / fibroblast proliferation / High laminar flow shear stress activates signaling by PIEZO1 and PECAM1:CDH5:KDR in endothelial cells / G alpha (i) signalling events / G alpha (s) signalling events / phospholipase C-activating G protein-coupled receptor signaling pathway / defense response to virus / G alpha (q) signalling events / response to ethanol / chemical synaptic transmission / perikaryon / Ras protein signal transduction / postsynaptic membrane / Extra-nuclear estrogen signaling / cell population proliferation Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.65 Å | |||||||||
 Authors Authors | Fay JF / Che T | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2023 Journal: Nature / Year: 2023Title: Ligand and G-protein selectivity in the κ-opioid receptor. Authors: Jianming Han / Jingying Zhang / Antonina L Nazarova / Sarah M Bernhard / Brian E Krumm / Lei Zhao / Jordy Homing Lam / Vipin A Rangari / Susruta Majumdar / David E Nichols / Vsevolod ...Authors: Jianming Han / Jingying Zhang / Antonina L Nazarova / Sarah M Bernhard / Brian E Krumm / Lei Zhao / Jordy Homing Lam / Vipin A Rangari / Susruta Majumdar / David E Nichols / Vsevolod Katritch / Peng Yuan / Jonathan F Fay / Tao Che /  Abstract: The κ-opioid receptor (KOR) represents a highly desirable therapeutic target for treating not only pain but also addiction and affective disorders. However, the development of KOR analgesics has ...The κ-opioid receptor (KOR) represents a highly desirable therapeutic target for treating not only pain but also addiction and affective disorders. However, the development of KOR analgesics has been hindered by the associated hallucinogenic side effects. The initiation of KOR signalling requires the G-family proteins including the conventional (G, G, G, G and G) and nonconventional (G and G) subtypes. How hallucinogens exert their actions through KOR and how KOR determines G-protein subtype selectivity are not well understood. Here we determined the active-state structures of KOR in a complex with multiple G-protein heterotrimers-G, G, G and G-using cryo-electron microscopy. The KOR-G-protein complexes are bound to hallucinogenic salvinorins or highly selective KOR agonists. Comparisons of these structures reveal molecular determinants critical for KOR-G-protein interactions as well as key elements governing G-family subtype selectivity and KOR ligand selectivity. Furthermore, the four G-protein subtypes display an intrinsically different binding affinity and allosteric activity on agonist binding at KOR. These results provide insights into the actions of opioids and G-protein-coupling specificity at KOR and establish a foundation to examine the therapeutic potential of pathway-selective agonists of KOR. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_27807.map.gz emd_27807.map.gz | 77.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-27807-v30.xml emd-27807-v30.xml emd-27807.xml emd-27807.xml | 26.4 KB 26.4 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_27807.png emd_27807.png | 41.2 KB | ||
| Filedesc metadata |  emd-27807.cif.gz emd-27807.cif.gz | 7.4 KB | ||
| Others |  emd_27807_additional_1.map.gz emd_27807_additional_1.map.gz emd_27807_half_map_1.map.gz emd_27807_half_map_1.map.gz emd_27807_half_map_2.map.gz emd_27807_half_map_2.map.gz | 85.2 MB 84.5 MB 84.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-27807 http://ftp.pdbj.org/pub/emdb/structures/EMD-27807 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27807 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27807 | HTTPS FTP |
-Related structure data
| Related structure data |  8dzsMC  8dzpC  8dzqC  8dzrC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_27807.map.gz / Format: CCP4 / Size: 91.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_27807.map.gz / Format: CCP4 / Size: 91.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | deep sharp | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.88 Å | ||||||||||||||||||||||||||||||||||||
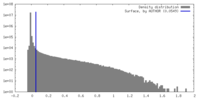
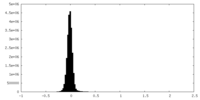
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: Bfact sharp
| File | emd_27807_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Bfact sharp | ||||||||||||
| Projections & Slices |
| ||||||||||||
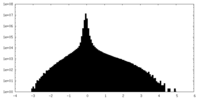
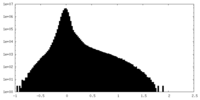
| Density Histograms |
-Half map: half A
| File | emd_27807_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half A | ||||||||||||
| Projections & Slices |
| ||||||||||||
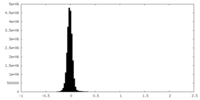
| Density Histograms |
-Half map: half B
| File | emd_27807_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half B | ||||||||||||
| Projections & Slices |
| ||||||||||||
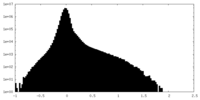
| Density Histograms |
- Sample components
Sample components
-Entire : GR89,696 bound Kappa Opioid Receptor in complex with Gz
| Entire | Name: GR89,696 bound Kappa Opioid Receptor in complex with Gz |
|---|---|
| Components |
|
-Supramolecule #1: GR89,696 bound Kappa Opioid Receptor in complex with Gz
| Supramolecule | Name: GR89,696 bound Kappa Opioid Receptor in complex with Gz type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#5 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Kappa-type opioid receptor
| Macromolecule | Name: Kappa-type opioid receptor / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 34.928555 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: LGSISPAIPV IITAVYSVVF VVGLVGNSLV MFVIIRYTKM KTATNIYIFN LALADALVTT TMPFQSTVYL MNSWPFGDVL CKIVLSIDY YNMFTSIFTL TMMSVDRYIA VCHPVKALDF RTPLKAKIIN ICIWLLSSSV GISAIVLGGT KVREDVDVIE C SLQFPDDD ...String: LGSISPAIPV IITAVYSVVF VVGLVGNSLV MFVIIRYTKM KTATNIYIFN LALADALVTT TMPFQSTVYL MNSWPFGDVL CKIVLSIDY YNMFTSIFTL TMMSVDRYIA VCHPVKALDF RTPLKAKIIN ICIWLLSSSV GISAIVLGGT KVREDVDVIE C SLQFPDDD YSWWDLFMKI CVFIFAFVIP VLIIIVCYTL MILRLKSVRL LSGSREKDRN LRRITRLVLV VVAVFVVCWT PI HIFILVE ALGSTSHSTA ALSSYYFCIA LGYTNSSLNP ILYAFLDENF KRCFRDFCFP LKMRMERQST S UniProtKB: Kappa-type opioid receptor |
-Macromolecule #2: Guanine nucleotide-binding protein G(z) subunit alpha
| Macromolecule | Name: Guanine nucleotide-binding protein G(z) subunit alpha / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 39.849551 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGSTVSAEDK AAAERSKMID KNLREDGEKQ RREIKLLLLG TSNSGKNTIV KQMKIIHSGG FNLEACKEYK PLIIYNAIDS LTRIIRALA ALRIDFHNPD RAYDAVQLFA LTGPAESKGE ITPELLGVMR RLWADPGAQA CFSRSSEYHL EDNAAYYLND L ERIAAADY ...String: MGSTVSAEDK AAAERSKMID KNLREDGEKQ RREIKLLLLG TSNSGKNTIV KQMKIIHSGG FNLEACKEYK PLIIYNAIDS LTRIIRALA ALRIDFHNPD RAYDAVQLFA LTGPAESKGE ITPELLGVMR RLWADPGAQA CFSRSSEYHL EDNAAYYLND L ERIAAADY IPTVEDILRS RDMTTGIVEN KFTFKELTFK MVDVGAQRSE RKKWIHCFEG VTAIIFCVEL SGYDLQTSRM AA SLKLFDS ICNNKWFIDT SLILFLNKKD LLAEKIRRIP LTICFPEYKG QNTYEEAAVY IQRQFEDLNR NKETKEIYSH FTC STDTSN IQFVFDAVTD VIIQNNLKYI GLC UniProtKB: Guanine nucleotide-binding protein G(z) subunit alpha |
-Macromolecule #3: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 37.285734 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: SELDQLRQEA EQLKNQIRDA RKACADATLS QITNNIDPVG RIQMRTRRTL RGHLAKIYAM HWGTDSRLLV SASQDGKLII WDSYTTNKV HAIPLRSSWV MTCAYAPSGN YVACGGLDNI CSIYNLKTRE GNVRVSRELA GHTGYLSCCR FLDDNQIVTS S GDTTCALW ...String: SELDQLRQEA EQLKNQIRDA RKACADATLS QITNNIDPVG RIQMRTRRTL RGHLAKIYAM HWGTDSRLLV SASQDGKLII WDSYTTNKV HAIPLRSSWV MTCAYAPSGN YVACGGLDNI CSIYNLKTRE GNVRVSRELA GHTGYLSCCR FLDDNQIVTS S GDTTCALW DIETGQQTTT FTGHTGDVMS LSLAPDTRLF VSGACDASAK LWDVREGMCR QTFTGHESDI NAICFFPNGN AF ATGSDDA TCRLFDLRAD QELMTYSHDN IICGITSVSF SKSGRLLLAG YDDFNCNVWD ALKADRAGVL AGHDNRVSCL GVT DDGMAV ATGSWDSFLK IWN UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 |
-Macromolecule #4: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 7.861143 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MASNNTASIA QARKLVEQLK MEANIDRIKV SKAAADLMAY CEAHAKEDPL LTPVPASENP FREKKFFCAI L UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 |
-Macromolecule #5: ScFv16 protein
| Macromolecule | Name: ScFv16 protein / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 26.679721 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: DVQLVESGGG LVQPGGSRKL SCSASGFAFS SFGMHWVRQA PEKGLEWVAY ISSGSGTIYY ADTVKGRFTI SRDDPKNTLF LQMTSLRSE DTAMYYCVRS IYYYGSSPFD FWGQGTTLTV SSGGGGSGGG GSGGGGSDIV MTQATSSVPV TPGESVSISC R SSKSLLHS ...String: DVQLVESGGG LVQPGGSRKL SCSASGFAFS SFGMHWVRQA PEKGLEWVAY ISSGSGTIYY ADTVKGRFTI SRDDPKNTLF LQMTSLRSE DTAMYYCVRS IYYYGSSPFD FWGQGTTLTV SSGGGGSGGG GSGGGGSDIV MTQATSSVPV TPGESVSISC R SSKSLLHS NGNTYLYWFL QRPGQSPQLL IYRMSNLASG VPDRFSGSGS GTAFTLTISR LEAEDVGVYY CMQHLEYPLT FG AGTKLEL KAAA |
-Macromolecule #6: methyl (3R)-4-[(3,4-dichlorophenyl)acetyl]-3-[(pyrrolidin-1-yl)me...
| Macromolecule | Name: methyl (3R)-4-[(3,4-dichlorophenyl)acetyl]-3-[(pyrrolidin-1-yl)methyl]piperazine-1-carboxylate type: ligand / ID: 6 / Number of copies: 1 / Formula: U9I |
|---|---|
| Molecular weight | Theoretical: 414.326 Da |
| Chemical component information | 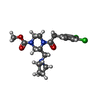 ChemComp-U9I: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE-PROPANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TALOS ARCTICA |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number real images: 3529 / Average electron dose: 43.8 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.1 µm |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller





































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)