+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Asp-bound GltPh RSMR mutant in iOFS state | |||||||||
 Map data Map data | Asp-bound GltPh RSMR mutant in iOFS state | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | GltPh / glutamate transporter / TRANSPORT PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationL-aspartate transmembrane transport / amino acid:sodium symporter activity / L-aspartate transmembrane transporter activity / L-aspartate import across plasma membrane / chloride transmembrane transporter activity / protein homotrimerization / chloride transmembrane transport / metal ion binding / identical protein binding / plasma membrane Similarity search - Function | |||||||||
| Biological species |   Pyrococcus horikoshii (archaea) Pyrococcus horikoshii (archaea) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.99 Å | |||||||||
 Authors Authors | Huang Y / Boudker O | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: J.Am.Chem.Soc. / Year: 2023 Journal: J.Am.Chem.Soc. / Year: 2023Title: Environmentally Ultrasensitive Fluorine Probe to Resolve Protein Conformational Ensembles by 19F NMR and Cryo-EM Authors: Huang Y / Reddy KD / Bracken C / Qiu B / Zhan W / Eliezer D / Boudker O | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_26504.map.gz emd_26504.map.gz | 83.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-26504-v30.xml emd-26504-v30.xml emd-26504.xml emd-26504.xml | 17.3 KB 17.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_26504_fsc.xml emd_26504_fsc.xml | 11.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_26504.png emd_26504.png | 99.6 KB | ||
| Filedesc metadata |  emd-26504.cif.gz emd-26504.cif.gz | 6.2 KB | ||
| Others |  emd_26504_half_map_1.map.gz emd_26504_half_map_1.map.gz emd_26504_half_map_2.map.gz emd_26504_half_map_2.map.gz | 154.6 MB 154.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-26504 http://ftp.pdbj.org/pub/emdb/structures/EMD-26504 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26504 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26504 | HTTPS FTP |
-Validation report
| Summary document |  emd_26504_validation.pdf.gz emd_26504_validation.pdf.gz | 993.2 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_26504_full_validation.pdf.gz emd_26504_full_validation.pdf.gz | 992.8 KB | Display | |
| Data in XML |  emd_26504_validation.xml.gz emd_26504_validation.xml.gz | 19.6 KB | Display | |
| Data in CIF |  emd_26504_validation.cif.gz emd_26504_validation.cif.gz | 24.8 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26504 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26504 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26504 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26504 | HTTPS FTP |
-Related structure data
| Related structure data |  7uh3MC  7ug0C  7ugdC  7ugjC  7uh6C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_26504.map.gz / Format: CCP4 / Size: 166.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_26504.map.gz / Format: CCP4 / Size: 166.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Asp-bound GltPh RSMR mutant in iOFS state | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.852 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Half Map 1
| File | emd_26504_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half Map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half Map 2
| File | emd_26504_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half Map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : GltPh RSMR mutant bound with Na and Asp
| Entire | Name: GltPh RSMR mutant bound with Na and Asp |
|---|---|
| Components |
|
-Supramolecule #1: GltPh RSMR mutant bound with Na and Asp
| Supramolecule | Name: GltPh RSMR mutant bound with Na and Asp / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:   Pyrococcus horikoshii (archaea) Pyrococcus horikoshii (archaea) |
-Macromolecule #1: Glutamate transporter homolog
| Macromolecule | Name: Glutamate transporter homolog / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Pyrococcus horikoshii (archaea) Pyrococcus horikoshii (archaea)Strain: ATCC 700860 / DSM 12428 / JCM 9974 / NBRC 100139 / OT-3 |
| Molecular weight | Theoretical: 44.13323 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGLYRKYIEY PVLQKILIGL ILGAIVGLIL GHYGYAHAVH TYVKPFGDLF VRLLKMLVMP IVFASLVVGA ASISPARLGR VGVKIVVYY LLTSAFAVTL GIIMARLFNP GAGIHLAVGG QQFQPHQAPP LVHILLDIVP TNPFGALANG QVLPTIFFAI I LGIAITYL ...String: MGLYRKYIEY PVLQKILIGL ILGAIVGLIL GHYGYAHAVH TYVKPFGDLF VRLLKMLVMP IVFASLVVGA ASISPARLGR VGVKIVVYY LLTSAFAVTL GIIMARLFNP GAGIHLAVGG QQFQPHQAPP LVHILLDIVP TNPFGALANG QVLPTIFFAI I LGIAITYL MNSENEKVRK SAETLLDAIN GLAEAMYKIV NGVMQYAPIG VFALIAHVMA HQGVHVVGEL AKVTAAVYVG LT LQILLVY FVLLKIYGID PISFIKHAKD AMLTAFVTSS SSGTLPVTMR VAKEMGISEG IYSFTLPLGA TINMDGTALY QGV ATFFIA NALGSHLTVG QQLTIVLTAV LASIGTAGVP GAGAIMLAMV LHSVGLPLTD PNVAAAYA(EFC)I LGIDAILDRG RTMVNVTGD LTGTAIVAKT EGT UniProtKB: Glutamate transporter homolog |
-Macromolecule #2: ASPARTIC ACID
| Macromolecule | Name: ASPARTIC ACID / type: ligand / ID: 2 / Number of copies: 1 / Formula: ASP |
|---|---|
| Molecular weight | Theoretical: 133.103 Da |
| Chemical component information | 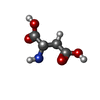 ChemComp-ASP: |
-Macromolecule #3: SODIUM ION
| Macromolecule | Name: SODIUM ION / type: ligand / ID: 3 / Number of copies: 3 |
|---|---|
| Molecular weight | Theoretical: 22.99 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 50.94 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.5 µm / Nominal defocus min: 1.3 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)






































