[English] 日本語
 Yorodumi
Yorodumi- EMDB-26169: Cryo-EM structure of human Anion Exchanger 1 bound to Niflumic Acid -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of human Anion Exchanger 1 bound to Niflumic Acid | ||||||||||||
 Map data Map data | Cryo-EM structure of human Anion Exchanger 1 bound to Niflumic Acid | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | Transmembrane / TRANSPORT PROTEIN | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationpH elevation / Defective SLC4A1 causes hereditary spherocytosis type 4 (HSP4), distal renal tubular acidosis (dRTA) and dRTA with hemolytic anemia (dRTA-HA) / negative regulation of urine volume / Bicarbonate transporters / intracellular monoatomic ion homeostasis / ankyrin-1 complex / plasma membrane phospholipid scrambling / monoatomic anion transmembrane transporter activity / chloride:bicarbonate antiporter activity / solute:inorganic anion antiporter activity ...pH elevation / Defective SLC4A1 causes hereditary spherocytosis type 4 (HSP4), distal renal tubular acidosis (dRTA) and dRTA with hemolytic anemia (dRTA-HA) / negative regulation of urine volume / Bicarbonate transporters / intracellular monoatomic ion homeostasis / ankyrin-1 complex / plasma membrane phospholipid scrambling / monoatomic anion transmembrane transporter activity / chloride:bicarbonate antiporter activity / solute:inorganic anion antiporter activity / bicarbonate transport / bicarbonate transmembrane transporter activity / monoatomic anion transport / chloride transport / chloride transmembrane transporter activity / ankyrin binding / negative regulation of glycolytic process through fructose-6-phosphate / hemoglobin binding / cortical cytoskeleton / erythrocyte development / protein-membrane adaptor activity / chloride transmembrane transport / protein localization to plasma membrane / regulation of intracellular pH / Erythrocytes take up oxygen and release carbon dioxide / Erythrocytes take up carbon dioxide and release oxygen / transmembrane transport / Z disc / cytoplasmic side of plasma membrane / blood coagulation / basolateral plasma membrane / blood microparticle / protein homodimerization activity / extracellular exosome / membrane / plasma membrane Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.18 Å | ||||||||||||
 Authors Authors | Capper MJ / Mathiharan YK | ||||||||||||
| Funding support |  United States, 3 items United States, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2023 Journal: Nat Struct Mol Biol / Year: 2023Title: Substrate binding and inhibition of the anion exchanger 1 transporter. Authors: Michael J Capper / Shifan Yang / Alexander C Stone / Sezen Vatansever / Gregory Zilberg / Yamuna Kalyani Mathiharan / Raul Habib / Keino Hutchinson / Yihan Zhao / Avner Schlessinger / Mihaly ...Authors: Michael J Capper / Shifan Yang / Alexander C Stone / Sezen Vatansever / Gregory Zilberg / Yamuna Kalyani Mathiharan / Raul Habib / Keino Hutchinson / Yihan Zhao / Avner Schlessinger / Mihaly Mezei / Roman Osman / Bin Zhang / Daniel Wacker /  Abstract: Anion exchanger 1 (AE1), a member of the solute carrier (SLC) family, is the primary bicarbonate transporter in erythrocytes, regulating pH levels and CO transport between lungs and tissues. Previous ...Anion exchanger 1 (AE1), a member of the solute carrier (SLC) family, is the primary bicarbonate transporter in erythrocytes, regulating pH levels and CO transport between lungs and tissues. Previous studies characterized its role in erythrocyte structure and provided insight into transport regulation. However, key questions remain regarding substrate binding and transport, mechanisms of drug inhibition and modulation by membrane components. Here we present seven cryo-EM structures in apo, bicarbonate-bound and inhibitor-bound states. These, combined with uptake and computational studies, reveal important molecular features of substrate recognition and transport, and illuminate sterol binding sites, to elucidate distinct inhibitory mechanisms of research chemicals and prescription drugs. We further probe the substrate binding site via structure-based ligand screening, identifying an AE1 inhibitor. Together, our findings provide insight into mechanisms of solute carrier transport and inhibition. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_26169.map.gz emd_26169.map.gz | 72.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-26169-v30.xml emd-26169-v30.xml emd-26169.xml emd-26169.xml | 22.6 KB 22.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_26169_fsc.xml emd_26169_fsc.xml | 11.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_26169.png emd_26169.png | 118 KB | ||
| Filedesc metadata |  emd-26169.cif.gz emd-26169.cif.gz | 7.3 KB | ||
| Others |  emd_26169_additional_1.map.gz emd_26169_additional_1.map.gz emd_26169_half_map_1.map.gz emd_26169_half_map_1.map.gz emd_26169_half_map_2.map.gz emd_26169_half_map_2.map.gz | 136.6 MB 134.2 MB 134.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-26169 http://ftp.pdbj.org/pub/emdb/structures/EMD-26169 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26169 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26169 | HTTPS FTP |
-Validation report
| Summary document |  emd_26169_validation.pdf.gz emd_26169_validation.pdf.gz | 786.3 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_26169_full_validation.pdf.gz emd_26169_full_validation.pdf.gz | 785.9 KB | Display | |
| Data in XML |  emd_26169_validation.xml.gz emd_26169_validation.xml.gz | 19.8 KB | Display | |
| Data in CIF |  emd_26169_validation.cif.gz emd_26169_validation.cif.gz | 25.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26169 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26169 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26169 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26169 | HTTPS FTP |
-Related structure data
| Related structure data |  7ty8MC  7ty4C  7ty6C  7ty7C  7tyaC  8t6uC  8t6vC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_26169.map.gz / Format: CCP4 / Size: 144.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_26169.map.gz / Format: CCP4 / Size: 144.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM structure of human Anion Exchanger 1 bound to Niflumic Acid | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.076 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: Sharpened map
| File | emd_26169_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half Map 1
| File | emd_26169_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half Map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half Map 2
| File | emd_26169_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half Map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Dimeric anion exchanger 1 (SLC4A1)
| Entire | Name: Dimeric anion exchanger 1 (SLC4A1) |
|---|---|
| Components |
|
-Supramolecule #1: Dimeric anion exchanger 1 (SLC4A1)
| Supramolecule | Name: Dimeric anion exchanger 1 (SLC4A1) / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 203 KDa |
-Macromolecule #1: Band 3 anion transport protein
| Macromolecule | Name: Band 3 anion transport protein / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 101.883859 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MEELQDDYED MMEENLEQEE YEDPDIPESQ MEEPAAHDTE ATATDYHTTS HPGTHKVYVE LQELVMDEKN QELRWMEAAR WVQLEENLG ENGAWGRPHL SHLTFWSLLE LRRVFTKGTV LLDLQETSLA GVANQLLDRF IFEDQIRPQD REELLRALLL K HSHAGELE ...String: MEELQDDYED MMEENLEQEE YEDPDIPESQ MEEPAAHDTE ATATDYHTTS HPGTHKVYVE LQELVMDEKN QELRWMEAAR WVQLEENLG ENGAWGRPHL SHLTFWSLLE LRRVFTKGTV LLDLQETSLA GVANQLLDRF IFEDQIRPQD REELLRALLL K HSHAGELE ALGGVKPAVL TRSGDPSQPL LPQHSSLETQ LFCEQGDGGT EGHSPSGILE KIPPDSEATL VLVGRADFLE QP VLGFVRL QEAAELEAVE LPVPIRFLFV LLGPEAPHID YTQLGRAAAT LMSERVFRID AYMAQSRGEL LHSLEGFLDC SLV LPPTDA PSEQALLSLV PVQRELLRRR YQSSPAKPDS SFYKGLDLNG GPDDPLQQTG QLFGGLVRDI RRRYPYYLSD ITDA FSPQV LAAVIFIYFA ALSPAITFGG LLGEKTRNQM GVSELLISTA VQGILFALLG AQPLLVVGFS GPLLVFEEAF FSFCE TNGL EYIVGRVWIG FWLILLVVLV VAFEGSFLVR FISRYTQEIF SFLISLIFIY ETFSKLIKIF QDHPLQKTYN YNVLMV PKP QGPLPNTALL SLVLMAGTFF FAMMLRKFKN SSYFPGKLRR VIGDFGVPIS ILIMVLVDFF IQDTYTQKLS VPDGFKV SN SSARGWVIHP LGLRSEFPIW MMFASALPAL LVFILIFLES QITTLIVSKP ERKMVKGSGF HLDLLLVVGM GGVAALFG M PWLSATTVRS VTHANALTVM GKASTPGAAA QIQEVKEQRI SGLLVAVLVG LSILMEPILS RIPLAVLFGI FLYMGVTSL SGIQLFDRIL LLFKPPKYHP DVPYVKRVKT WRMHLFTGIQ IICLAVLWVV KSTPASLALP FVLILTVPLR RVLLPLIFRN VELQCLDAD DAKATFDEEE GRDEYDEVAM PV UniProtKB: Band 3 anion transport protein |
-Macromolecule #3: CHOLESTEROL
| Macromolecule | Name: CHOLESTEROL / type: ligand / ID: 3 / Number of copies: 8 / Formula: CLR |
|---|---|
| Molecular weight | Theoretical: 386.654 Da |
| Chemical component information |  ChemComp-CLR: |
-Macromolecule #4: 1,2-DIACYL-SN-GLYCERO-3-PHOSPHOCHOLINE
| Macromolecule | Name: 1,2-DIACYL-SN-GLYCERO-3-PHOSPHOCHOLINE / type: ligand / ID: 4 / Number of copies: 2 / Formula: PC1 |
|---|---|
| Molecular weight | Theoretical: 790.145 Da |
| Chemical component information |  ChemComp-PC1: |
-Macromolecule #5: 2-{[3-(TRIFLUOROMETHYL)PHENYL]AMINO}NICOTINIC ACID
| Macromolecule | Name: 2-{[3-(TRIFLUOROMETHYL)PHENYL]AMINO}NICOTINIC ACID / type: ligand / ID: 5 / Number of copies: 2 / Formula: NFL |
|---|---|
| Molecular weight | Theoretical: 282.218 Da |
| Chemical component information | 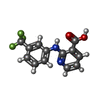 ChemComp-NFL: |
-Macromolecule #6: water
| Macromolecule | Name: water / type: ligand / ID: 6 / Number of copies: 29 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 5 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
| ||||||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Chamber temperature: 285 K / Instrument: LEICA EM GP Details: Blot force 3 for 3-5 seconds was used and subsequent grids were screened for ice thickness prior to data collection. | ||||||||||||
| Details | The sample was monodisperse following gel filtration and immediately concentrated for grid preparation |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 1 / Number real images: 5274 / Average electron dose: 59.99 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 1.8 µm / Nominal defocus min: 0.5 µm / Nominal magnification: 64000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)