+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-24178 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of human NPC1L1 | |||||||||
 Map data Map data | Structure of human NPC1L1 | |||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationIntestinal lipid absorption / cellular response to sterol depletion / vitamin E binding / vitamin E metabolic process / vitamin transport / intestinal cholesterol absorption / lipoprotein metabolic process / myosin V binding / cholesterol transport / cholesterol binding ...Intestinal lipid absorption / cellular response to sterol depletion / vitamin E binding / vitamin E metabolic process / vitamin transport / intestinal cholesterol absorption / lipoprotein metabolic process / myosin V binding / cholesterol transport / cholesterol binding / cholesterol biosynthetic process / cholesterol homeostasis / cytoplasmic vesicle membrane / small GTPase binding / apical plasma membrane / protein homodimerization activity / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.34 Å | |||||||||
 Authors Authors | Li X / Long T | |||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2021 Journal: Sci Adv / Year: 2021Title: Structures of dimeric human NPC1L1 provide insight into mechanisms for cholesterol absorption. Authors: Tao Long / Yang Liu / Yu Qin / Russell A DeBose-Boyd / Xiaochun Li /  Abstract: Polytopic Niemann-Pick C1-like 1 (NPC1L1) plays a major role in intestinal absorption of biliary cholesterol, vitamin E (VE), and vitamin K (VK). The drug ezetimibe inhibits NPC1L1-mediated ...Polytopic Niemann-Pick C1-like 1 (NPC1L1) plays a major role in intestinal absorption of biliary cholesterol, vitamin E (VE), and vitamin K (VK). The drug ezetimibe inhibits NPC1L1-mediated absorption of cholesterol, lowering of circulating levels of low-density lipoprotein cholesterol. Here, we report cryo-electron microscopy structures of human NPC1L1 (hNPC1L1) bound to either cholesterol or a lipid resembling VE. These findings, together with functional assays, reveal that the same intramolecular channel in hNPC1L1 mediates transport of VE and cholesterol. hNPC1L1 exists primarily as a homodimer; dimerization is mediated by aromatic residues within a region of transmembrane helix 2 that exhibits a horizonal orientation in the membrane. Mutation of tryptophan-347 lies in this region disrupts dimerization and the resultant monomeric NPC1L1 exhibits reduced efficiency of cholesterol uptake. These findings identify the oligomeric state of hNPC1L1 as a target for therapies that inhibit uptake of dietary cholesterol and reduce the incidence of cardiovascular disease. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_24178.map.gz emd_24178.map.gz | 168 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-24178-v30.xml emd-24178-v30.xml emd-24178.xml emd-24178.xml | 11 KB 11 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_24178.png emd_24178.png | 73.9 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-24178 http://ftp.pdbj.org/pub/emdb/structures/EMD-24178 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24178 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24178 | HTTPS FTP |
-Validation report
| Summary document |  emd_24178_validation.pdf.gz emd_24178_validation.pdf.gz | 427.3 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_24178_full_validation.pdf.gz emd_24178_full_validation.pdf.gz | 426.9 KB | Display | |
| Data in XML |  emd_24178_validation.xml.gz emd_24178_validation.xml.gz | 7 KB | Display | |
| Data in CIF |  emd_24178_validation.cif.gz emd_24178_validation.cif.gz | 7.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24178 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24178 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24178 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24178 | HTTPS FTP |
-Related structure data
| Related structure data |  7n4uMC  7n4vC  7n4xC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_24178.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_24178.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Structure of human NPC1L1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.833 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
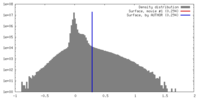
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : NPC1L1 apo state
| Entire | Name: NPC1L1 apo state |
|---|---|
| Components |
|
-Supramolecule #1: NPC1L1 apo state
| Supramolecule | Name: NPC1L1 apo state / type: cell / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Isoform 2 of NPC1-like intracellular cholesterol transporter 1
| Macromolecule | Name: Isoform 2 of NPC1-like intracellular cholesterol transporter 1 type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 145.904031 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MAEAGLRGWL LWALLLRLAQ SEPYTTIHQP GYCAFYDECG KNPELSGSLM TLSNVSCLSN TPARKITGDH LILLQKICPR LYTGPNTQA CCSAKQLVSL EASLSITKAL LTRCPACSDN FVNLHCHNTC SPNQSLFINV TRVAQLGAGQ LPAVVAYEAF Y QHSFAEQS ...String: MAEAGLRGWL LWALLLRLAQ SEPYTTIHQP GYCAFYDECG KNPELSGSLM TLSNVSCLSN TPARKITGDH LILLQKICPR LYTGPNTQA CCSAKQLVSL EASLSITKAL LTRCPACSDN FVNLHCHNTC SPNQSLFINV TRVAQLGAGQ LPAVVAYEAF Y QHSFAEQS YDSCSRVRVP AAATLAVGTM CGVYGSALCN AQRWLNFQGD TGNGLAPLDI TFHLLEPGQA VGSGIQPLNE GV ARCNESQ GDDVATCSCQ DCAASCPAIA RPQALDSTFY LGQMPGSLVL IIILCSVFAV VTILLVGFRV APARDKSKMV DPK KGTSLS DKLSFSTHTL LGQFFQGWGT WVASWPLTIL VLSVIPVVAL AAGLVFTELT TDPVELWSAP NSQARSEKAF HDQH FGPFF RTNQVILTAP NRSSYRYDSL LLGPKNFSGI LDLDLLLELL ELQERLRHLQ VWSPEAQRNI SLQDICYAPL NPDNT SLYD CCINSLLQYF QNNRTLLLLT ANQTLMGQTS QVDWKDHFLY CANAPLTFKD GTALALSCMA DYGAPVFPFL AIGGYK GKD YSEAEALIMT FSLNNYPAGD PRLAQAKLWE EAFLEEMRAF QRRMAGMFQV TFMAERSLED EINRTTAEDL PIFATSY IV IFLYISLALG SYSSWSRVMV DSKATLGLGG VAVVLGAVMA AMGFFSYLGI RSSLVILQVV PFLVLSVGAD NIFIFVLE Y QRLPRRPGEP REVHIGRALG RVAPSMLLCS LSEAICFFLG ALTPMPAVRT FALTSGLAVI LDFLLQMSAF VALLSLDSK RQEASRLDVC CCVKPQELPP PGQGEGLLLG FFQKAYAPFL LHWITRGVVL LLFLALFGVS LYSMCHISVG LDQELALPKD SYLLDYFLF LNRYFEVGAP VYFVTTLGYN FSSEAGMNAI CSSAGCNNFS FTQKIQYATE FPEQSYLAIP ASSWVDDFID W LTPSSCCR LYISGPNKDK FCPSTVNSLN CLKNCMSITM GSVRPSVEQF HKYLPWFLND RPNIKCPKGG LAAYSTSVNL TS DGQVLTS RFMAYHKPLK NSQDYTEALR AARELAANIT ADLRKVPGTD PAFEVFPYTI TNVFYEQYLT ILPEGLFMLS LCL VPTFAV SCLLLGLDLR SGLLNLLSIV MILVDTVGFM ALWGISYNAV SLINLVSAVG MSVEFVSHIT RSFAISTKPT WLER AKEAT ISMGSAVFAG VAMTNLPGIL VLGLAKAQLI QIFFFRLNLL ITLLGLLHGL VFLPVILSYV GPDVNPALAL EQKRA EEAV AAVMVASCPN HPSRVSTADN IYVNHSFEGS IKGAGAISNF LPNNGRQF |
-Macromolecule #3: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 3 / Number of copies: 8 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Macromolecule #4: CHOLESTEROL
| Macromolecule | Name: CHOLESTEROL / type: ligand / ID: 4 / Number of copies: 4 / Formula: CLR |
|---|---|
| Molecular weight | Theoretical: 386.654 Da |
| Chemical component information |  ChemComp-CLR: |
-Macromolecule #5: (2R)-2,5,7,8-TETRAMETHYL-2-[(4R,8R)-4,8,12-TRIMETHYLTRIDECYL]CHRO...
| Macromolecule | Name: (2R)-2,5,7,8-TETRAMETHYL-2-[(4R,8R)-4,8,12-TRIMETHYLTRIDECYL]CHROMAN-6-OL type: ligand / ID: 5 / Number of copies: 2 / Formula: VIV |
|---|---|
| Molecular weight | Theoretical: 430.706 Da |
| Chemical component information | 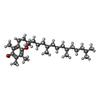 ChemComp-VIV: |
-Macromolecule #6: (2S)-3-(hexadecanoyloxy)-2-[(9Z)-octadec-9-enoyloxy]propyl 2-(tri...
| Macromolecule | Name: (2S)-3-(hexadecanoyloxy)-2-[(9Z)-octadec-9-enoyloxy]propyl 2-(trimethylammonio)ethyl phosphate type: ligand / ID: 6 / Number of copies: 2 / Formula: POV |
|---|---|
| Molecular weight | Theoretical: 760.076 Da |
| Chemical component information |  ChemComp-POV: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: DARK FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.34 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 135911 |
|---|---|
| Initial angle assignment | Type: ANGULAR RECONSTITUTION |
| Final angle assignment | Type: ANGULAR RECONSTITUTION |
 Movie
Movie Controller
Controller














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)