[English] 日本語
 Yorodumi
Yorodumi- EMDB-22987: Adeno-associated virus serotype 5 at 2.1 Angstroms resolution, AAV5 -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-22987 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Adeno-associated virus serotype 5 at 2.1 Angstroms resolution, AAV5 | |||||||||
 Map data Map data | 2.1A Native AAV5 virus like particle sharpened map. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | AAV5 / AAV / AAV-5 / Adeno Associated Virus / VIRUS LIKE PARTICLE / parvovirus / virus / gene therapy | |||||||||
| Function / homology | Phospholipase A2-like domain / Phospholipase A2-like domain / Parvovirus coat protein VP2 / Parvovirus coat protein VP1/VP2 / Parvovirus coat protein VP1/VP2 / Capsid/spike protein, ssDNA virus / T=1 icosahedral viral capsid / structural molecule activity / Capsid protein Function and homology information Function and homology information | |||||||||
| Biological species |  Adeno-associated virus - 5 / Adeno-associated virus - 5 /   Adeno-associated virus Adeno-associated virus | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.1 Å | |||||||||
 Authors Authors | Silveria M / Chapman MS | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Viruses / Year: 2020 Journal: Viruses / Year: 2020Title: The Structure of an AAV5-AAVR Complex at 2.5 Å Resolution: Implications for Cellular Entry and Immune Neutralization of AAV Gene Therapy Vectors. Authors: Mark A Silveria / Edward E Large / Grant M Zane / Tommi A White / Michael S Chapman /  Abstract: Adeno-Associated Virus is the leading vector for gene therapy. Although it is the vector for all in vivo gene therapies approved for clinical use by the US Food and Drug Administration, its biology ...Adeno-Associated Virus is the leading vector for gene therapy. Although it is the vector for all in vivo gene therapies approved for clinical use by the US Food and Drug Administration, its biology is still not yet fully understood. It has been shown that different serotypes of AAV bind to their cellular receptor, AAVR, in different ways. Previously we have reported a 2.4Å structure of AAV2 bound to AAVR that shows ordered structure for only one of the two AAVR domains with which AAV2 interacts. In this study we present a 2.5Å resolution structure of AAV5 bound to AAVR. AAV5 binds to the first polycystic kidney disease (PKD) domain of AAVR that was not ordered in the AAV2 structure. Interactions of AAV5 with AAVR are analyzed in detail, and the implications for AAV2 binding are explored through molecular modeling. Moreover, we find that binding sites for the antibodies ADK5a, ADK5b, and 3C5 on AAV5 overlap with the binding site of AAVR. These insights provide a structural foundation for development of gene therapy agents to better evade immune neutralization without disrupting cellular entry. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_22987.map.gz emd_22987.map.gz | 203.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-22987-v30.xml emd-22987-v30.xml emd-22987.xml emd-22987.xml | 22 KB 22 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_22987.png emd_22987.png | 257.9 KB | ||
| Masks |  emd_22987_msk_1.map emd_22987_msk_1.map | 729 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-22987.cif.gz emd-22987.cif.gz | 7.1 KB | ||
| Others |  emd_22987_additional_1.map.gz emd_22987_additional_1.map.gz emd_22987_half_map_1.map.gz emd_22987_half_map_1.map.gz emd_22987_half_map_2.map.gz emd_22987_half_map_2.map.gz | 576.9 MB 577.2 MB 577.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-22987 http://ftp.pdbj.org/pub/emdb/structures/EMD-22987 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22987 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22987 | HTTPS FTP |
-Related structure data
| Related structure data |  7kp3MC  7kpnC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_22987.map.gz / Format: CCP4 / Size: 729 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_22987.map.gz / Format: CCP4 / Size: 729 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | 2.1A Native AAV5 virus like particle sharpened map. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.5325 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_22987_msk_1.map emd_22987_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
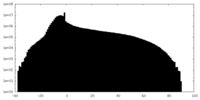
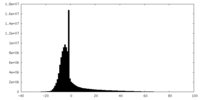
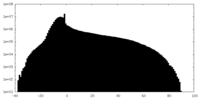
| Density Histograms |
-Additional map: 2.1A Native AAV5 virus like particle unsharpened map.
| File | emd_22987_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | 2.1A Native AAV5 virus like particle unsharpened map. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: 2.1A Native AAV5 virus like particle sharpened map.
| File | emd_22987_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | 2.1A Native AAV5 virus like particle sharpened map. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: 2.1A Native AAV5 virus like particle half map...
| File | emd_22987_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | 2.1A Native AAV5 virus like particle half map 2 of 2 in 222a viewing frame. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Adeno-associated virus
| Entire | Name:   Adeno-associated virus Adeno-associated virus |
|---|---|
| Components |
|
-Supramolecule #1: Adeno-associated virus
| Supramolecule | Name: Adeno-associated virus / type: virus / ID: 1 / Parent: 0 / Macromolecule list: all Details: Expressed using SF9 cells with a pfastbac LIC vector. Purified with cesium chloride ultracentrifugation. NCBI-ID: 272636 / Sci species name: Adeno-associated virus / Virus type: VIRUS-LIKE PARTICLE / Virus isolate: STRAIN / Virus enveloped: No / Virus empty: Yes |
|---|---|
| Host (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 3.746 MDa |
| Virus shell | Shell ID: 1 / Diameter: 250.0 Å / T number (triangulation number): 1 |
-Macromolecule #1: Capsid protein
| Macromolecule | Name: Capsid protein / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Adeno-associated virus - 5 Adeno-associated virus - 5 |
| Molecular weight | Theoretical: 80.366211 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: SFVDHPPDWL EEVGEGLREF LGLEAGPPKP KPNQQHQDQA RGLVLPGYNY LGPGNGLDRG EPVNRADEVA REHDISYNEQ LEAGDNPYL KYNHADAEFQ EKLADDTSFG GNLGKAVFQA KKRVLEPFGL VEEGAKTAPT GKRIDDHFPK RKKARTEEDS K PSTSSDAE ...String: SFVDHPPDWL EEVGEGLREF LGLEAGPPKP KPNQQHQDQA RGLVLPGYNY LGPGNGLDRG EPVNRADEVA REHDISYNEQ LEAGDNPYL KYNHADAEFQ EKLADDTSFG GNLGKAVFQA KKRVLEPFGL VEEGAKTAPT GKRIDDHFPK RKKARTEEDS K PSTSSDAE AGPSGSQQLQ IPAQPASSLG ADTMSAGGGG PLGDNNQGAD GVGNASGDWH CDSTWMGDRV VTKSTRTWVL PS YNNHQYR EIKSGSVDGS NANAYFGYST PWGYFDFNRF HSHWSPRDWQ RLINNYWGFR PRSLRVKIFN IQVKEVTVQD STT TIANNL TSTVQVFTDD DYQLPYVVGN GTEGCLPAFP PQVFTLPQYG YATLNRDNTE NPTERSSFFC LEYFPSKMLR TGNN FEFTY NFEEVPFHSS FAPSQNLFKL ANPLVDQYLY RFVSTNNTGG VQFNKNLAGR YANTYKNWFP GPMGRTQGWN LGSGV NRAS VSAFATTNRM ELEGASYQVP PQPNGMTNNL QGSNTYALEN TMIFNSQPAN PGTTATYLEG NMLITSESET QPVNRV AYN VGGQMATNNQ SSTTAPATGT YNLQEIVPGS VWMERDVYLQ GPIWAKIPET GAHFHPSPAM GGFGLKHPPP MMLIKNT PV PGNITSFSDV PVSSFITQYS TGQVTVEMEW ELKKENSKRW NPEIQYTNNY NDPQFVDFAP DSTGEYRTTR PIGTRYLT R PL UniProtKB: Capsid protein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.75 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.4 Component:
| ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 293 K / Instrument: FEI VITROBOT MARK IV Details: Two 2uL aliquots applied to grid (manual blotting between), prior to automated 3 second blot before plunging.. | ||||||||||||
| Details | Monodisperse |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Temperature | Min: 93.0 K / Max: 93.0 K |
| Details | Coma-free alignment and objective astigmatism where corrected using Sherpa (Thermo Fisher, Inc.). |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Detector mode: INTEGRATING / Digitization - Dimensions - Width: 11520 pixel / Digitization - Dimensions - Height: 8184 pixel / Number grids imaged: 1 / Number real images: 9490 / Average electron dose: 31.7 e/Å2 / Details: Pixel size was 0.5295 angstrom. |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: -2.7 µm / Nominal defocus min: -0.7000000000000001 µm / Nominal magnification: 81000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Source name: PDB / Chain - Initial model type: experimental model |
|---|---|
| Details | Stand-alone RSRef was used for refinement of magnification, resolution, envelope correction and atomic B-factors. This was alternated with RSRef-embedded CNS was used for molecular dynamics optimization (1st round) and stereochemically-restrained all-atom least-squares optimization. |
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT / Target criteria: Least-squares residual |
| Output model |  PDB-7kp3: |
 Movie
Movie Controller
Controller
















 Z (Sec.)
Z (Sec.) X (Row.)
X (Row.) Y (Col.)
Y (Col.)