+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-22390 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Sheep Connexin-50 at 2.5 angstroms reoslution, Lipid Class 2 | |||||||||
 Map data Map data | Local resolution-filtered map. Used for model-building. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Connexin / Gap Junction / Lipid / Nanodisc / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationgap junction-mediated intercellular transport / gap junction hemi-channel activity / connexin complex / gap junction channel activity / visual perception / cell-cell signaling / plasma membrane Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.5 Å | |||||||||
 Authors Authors | Flores JA / Haddad BG | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2020 Journal: Nat Commun / Year: 2020Title: Connexin-46/50 in a dynamic lipid environment resolved by CryoEM at 1.9 Å. Authors: Jonathan A Flores / Bassam G Haddad / Kimberly A Dolan / Janette B Myers / Craig C Yoshioka / Jeremy Copperman / Daniel M Zuckerman / Steve L Reichow /  Abstract: Gap junctions establish direct pathways for cells to transfer metabolic and electrical messages. The local lipid environment is known to affect the structure, stability and intercellular channel ...Gap junctions establish direct pathways for cells to transfer metabolic and electrical messages. The local lipid environment is known to affect the structure, stability and intercellular channel activity of gap junctions; however, the molecular basis for these effects remains unknown. Here, we incorporate native connexin-46/50 (Cx46/50) intercellular channels into a dual lipid nanodisc system, mimicking a native cell-to-cell junction. Structural characterization by CryoEM reveals a lipid-induced stabilization to the channel, resulting in a 3D reconstruction at 1.9 Å resolution. Together with all-atom molecular dynamics simulations, it is shown that Cx46/50 in turn imparts long-range stabilization to the dynamic local lipid environment that is specific to the extracellular lipid leaflet. In addition, ~400 water molecules are resolved in the CryoEM map, localized throughout the intercellular permeation pathway and contributing to the channel architecture. These results illustrate how the aqueous-lipid environment is integrated with the architectural stability, structure and function of gap junction communication channels. #1:  Journal: BioRxiv / Year: 2020 Journal: BioRxiv / Year: 2020Title: Connexin-46/50 in a dynamic lipid environment resolved by CryoEM at 1.9 angstroms Authors: Flores JA / Haddad BG / Dolan KA / Myers JA / Yoshioka CC / Copperman J / Zuckerman DM / Reichow SL | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_22390.map.gz emd_22390.map.gz | 154 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-22390-v30.xml emd-22390-v30.xml emd-22390.xml emd-22390.xml | 26.9 KB 26.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_22390_fsc.xml emd_22390_fsc.xml | 14.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_22390.png emd_22390.png | 162.3 KB | ||
| Masks |  emd_22390_msk_1.map emd_22390_msk_1.map | 244.1 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-22390.cif.gz emd-22390.cif.gz | 6.1 KB | ||
| Others |  emd_22390_additional_1.map.gz emd_22390_additional_1.map.gz emd_22390_additional_2.map.gz emd_22390_additional_2.map.gz emd_22390_additional_3.map.gz emd_22390_additional_3.map.gz emd_22390_additional_4.map.gz emd_22390_additional_4.map.gz emd_22390_half_map_1.map.gz emd_22390_half_map_1.map.gz emd_22390_half_map_2.map.gz emd_22390_half_map_2.map.gz | 228.7 MB 20 MB 226.2 MB 191.8 MB 192.1 MB 192.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-22390 http://ftp.pdbj.org/pub/emdb/structures/EMD-22390 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22390 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22390 | HTTPS FTP |
-Related structure data
| Related structure data |  7jm9MC  7jn0MC  7jjpC  7jkcC  7jlwC  7jmcC  7jmdC  7jn1C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10480 (Title: Connexin-46/50 in a dynamic lipid environment resolved by CryoEM at 1.9 Å EMPIAR-10480 (Title: Connexin-46/50 in a dynamic lipid environment resolved by CryoEM at 1.9 ÅData size: 4.1 TB Data #1: Unaligned multiframe micrographs of sheep lens Connexin-46/50 gap junctions embedded in MSP1E1 lipid nanodiscs [micrographs - multiframe]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_22390.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_22390.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Local resolution-filtered map. Used for model-building. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.649 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_22390_msk_1.map emd_22390_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Postprocessed map, unmasked.
| File | emd_22390_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Postprocessed map, unmasked. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Postprocessed map, masked.
| File | emd_22390_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Postprocessed map, masked. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Postprocessed map, lowpass-filtered to 3.5 angstroms. Used to...
| File | emd_22390_additional_3.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Postprocessed map, lowpass-filtered to 3.5 angstroms. Used to aid lipid modeling. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Pre-postprocessed map.
| File | emd_22390_additional_4.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Pre-postprocessed map. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half-map (even) used for calculation of pre-postprocessed, postprocessed,...
| File | emd_22390_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half-map (even) used for calculation of pre-postprocessed, postprocessed, and local resolution-filtered maps. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half-map (odd) used for calculation of pre-postprocessed, postprocessed,...
| File | emd_22390_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half-map (odd) used for calculation of pre-postprocessed, postprocessed, and local resolution-filtered maps. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Dodecameric Connexin-50 Gap Junction Channel Complex
| Entire | Name: Dodecameric Connexin-50 Gap Junction Channel Complex |
|---|---|
| Components |
|
-Supramolecule #1: Dodecameric Connexin-50 Gap Junction Channel Complex
| Supramolecule | Name: Dodecameric Connexin-50 Gap Junction Channel Complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 450 KDa |
-Macromolecule #1: Gap junction alpha-8 protein
| Macromolecule | Name: Gap junction alpha-8 protein / type: protein_or_peptide / ID: 1 / Number of copies: 12 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 49.216809 KDa |
| Sequence | String: MGDWSFLGNI LEEVNEHSTV IGRVWLTVLF IFRILILGTA AEFVWGDEQS DFVCNTQQPG CENVCYDEAF PISHIRLWVL QIIFVSTPS LVYVGHAVHH VRMEEKRKER EAEELSQQSP GNGGERAPLA ADQGSVKKSS SSSKGTKKFR LEGTLLRTYV C HIIFKTLF ...String: MGDWSFLGNI LEEVNEHSTV IGRVWLTVLF IFRILILGTA AEFVWGDEQS DFVCNTQQPG CENVCYDEAF PISHIRLWVL QIIFVSTPS LVYVGHAVHH VRMEEKRKER EAEELSQQSP GNGGERAPLA ADQGSVKKSS SSSKGTKKFR LEGTLLRTYV C HIIFKTLF EVGFIVGHYF LYGFRILPLY RCSRWPCPNV VDCFVSRPTE KTIFILFMLS VASVSLFLNI LEMSHLGLKK IR SAFKRPV EQPLGEIPEK SLHSIAVSSI QKAKGYQLLE EEKIVSHYFP LTEVGMVEAS PLSAKPFSQF EEKVGPGPLG DLS RAYQET LPSYAQVGAQ EGVEEEQPIE AAAEPEVGDK SQEAERVSTE GEETLAVLEE EKVEPPEVEK EAEKEETPPE KVSK QELTP EKAPSLCAEL PGEDTRPLSR LSKASSRARS DDLTV UniProtKB: Gap junction alpha-8 protein |
-Macromolecule #2: 1,2-DIMYRISTOYL-RAC-GLYCERO-3-PHOSPHOCHOLINE
| Macromolecule | Name: 1,2-DIMYRISTOYL-RAC-GLYCERO-3-PHOSPHOCHOLINE / type: ligand / ID: 2 / Number of copies: 168 / Formula: MC3 |
|---|---|
| Molecular weight | Theoretical: 677.933 Da |
| Chemical component information | 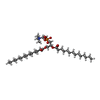 ChemComp-MC3: |
-Macromolecule #3: water
| Macromolecule | Name: water / type: ligand / ID: 3 / Number of copies: 156 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Support film - Material: CARBON / Support film - topology: HOLEY |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: COUNTING / Digitization - Dimensions - Width: 4096 pixel / Digitization - Dimensions - Height: 4096 pixel / Number grids imaged: 1 / Number real images: 2088 / Average exposure time: 30.0 sec. / Average electron dose: 52.5 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)