+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-22185 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | CueR-transcription activation complex with RNA transcript | |||||||||
 Map data Map data | CueR-transcription activation complex with RNA transcript | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Transcription activation / RNA polymerase / MerR-family / TRANSCRIPTION / TRANSCRIPTION-DNA-RNA complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationsigma factor antagonist complex / DNA-binding transcription activator activity / cytosolic DNA-directed RNA polymerase complex / regulation of DNA-templated transcription initiation / sigma factor activity / cis-regulatory region sequence-specific DNA binding / DNA-directed RNA polymerase complex / DNA-templated transcription initiation / protein-DNA complex / ribonucleoside binding ...sigma factor antagonist complex / DNA-binding transcription activator activity / cytosolic DNA-directed RNA polymerase complex / regulation of DNA-templated transcription initiation / sigma factor activity / cis-regulatory region sequence-specific DNA binding / DNA-directed RNA polymerase complex / DNA-templated transcription initiation / protein-DNA complex / ribonucleoside binding / DNA-directed 5'-3' RNA polymerase activity / DNA-directed RNA polymerase / response to heat / transcription cis-regulatory region binding / protein dimerization activity / copper ion binding / DNA-binding transcription factor activity / negative regulation of DNA-templated transcription / DNA-templated transcription / positive regulation of DNA-templated transcription / magnesium ion binding / DNA binding / zinc ion binding / identical protein binding / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.1 Å | |||||||||
 Authors Authors | Liu B / Shi W | |||||||||
 Citation Citation |  Journal: iScience / Year: 2021 Journal: iScience / Year: 2021Title: Structural basis of copper-efflux-regulator-dependent transcription activation. Authors: Wei Shi / Baoyue Zhang / Yanan Jiang / Chang Liu / Wei Zhou / Ming Chen / Yang Yang / Yangbo Hu / Bin Liu /   Abstract: The copper efflux regulator (CueR), a representative member of mercury resistance regulator (MerR) family metalloregulators, controls expression of copper homeostasis-regulating genes in bacteria. ...The copper efflux regulator (CueR), a representative member of mercury resistance regulator (MerR) family metalloregulators, controls expression of copper homeostasis-regulating genes in bacteria. The mechanism of transcription activation by CueR and other MerR family regulators is bending the spacer domain of promoter DNA. Here, we report the cryo-EM structures of the intact CueR-dependent transcription activation complexes. The structures show that CueR dimer bends the 19-bp promoter spacer to realign the -35 and -10 elements for recognition by σ-RNA polymerase holoenzyme and reveal a previously unreported interaction between the DNA-binding domain (DBD) from one CueR subunit and the σ nonconserved region (σNCR). Functional studies have shown that the CueR-σNCR interaction plays an auxiliary role in CueR-dependent transcription, assisting the activation mechanism of bending promoter DNA by CueR dimer. Because DBDs are highly conserved in sequence and structure, this transcription-activating mechanism could be generally used by MerR family regulators. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_22185.map.gz emd_22185.map.gz | 171.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-22185-v30.xml emd-22185-v30.xml emd-22185.xml emd-22185.xml | 24.7 KB 24.7 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_22185.png emd_22185.png | 76.5 KB | ||
| Filedesc metadata |  emd-22185.cif.gz emd-22185.cif.gz | 8.7 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-22185 http://ftp.pdbj.org/pub/emdb/structures/EMD-22185 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22185 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22185 | HTTPS FTP |
-Validation report
| Summary document |  emd_22185_validation.pdf.gz emd_22185_validation.pdf.gz | 587.1 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_22185_full_validation.pdf.gz emd_22185_full_validation.pdf.gz | 586.7 KB | Display | |
| Data in XML |  emd_22185_validation.xml.gz emd_22185_validation.xml.gz | 7 KB | Display | |
| Data in CIF |  emd_22185_validation.cif.gz emd_22185_validation.cif.gz | 8 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-22185 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-22185 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-22185 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-22185 | HTTPS FTP |
-Related structure data
| Related structure data |  6xh8MC  6xh7C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_22185.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_22185.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | CueR-transcription activation complex with RNA transcript | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.89 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
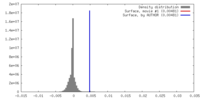
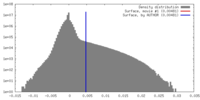
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
+Entire : CueR-TAC with RNA
+Supramolecule #1: CueR-TAC with RNA
+Macromolecule #1: DNA-directed RNA polymerase subunit alpha
+Macromolecule #2: DNA-directed RNA polymerase subunit beta
+Macromolecule #3: DNA-directed RNA polymerase subunit beta'
+Macromolecule #4: DNA-directed RNA polymerase subunit omega
+Macromolecule #5: RNA polymerase sigma factor RpoD
+Macromolecule #6: HTH-type transcriptional regulator CueR
+Macromolecule #7: NONTEMPLATE STRAND DNA (54-MER)
+Macromolecule #8: TEMPLATE STRAND DNA (54-MER)
+Macromolecule #9: NASCENT RNA
+Macromolecule #10: ZINC ION
+Macromolecule #11: COPPER (II) ION
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.5 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Grid | Model: Quantifoil R2/2 / Material: COPPER / Mesh: 200 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 60 sec. |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: COUNTING / Number grids imaged: 1 / Number real images: 4498 / Average exposure time: 30.0 sec. / Average electron dose: 30.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Calibrated defocus max: 2.4 µm / Calibrated defocus min: 1.0 µm / Calibrated magnification: 96000 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.4 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 96000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller



















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)