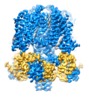
登録情報 データベース : EMDB / ID : EMD-20965タイトル structure of human KCNQ1-CaM complex human KCNQ1 複合体 : KCNQ1-CaM complexタンパク質・ペプチド : Potassium voltage-gated channel subfamily KQT member 1タンパク質・ペプチド : Calmodulin-1リガンド : CALCIUM ION / / / 機能・相同性 分子機能 ドメイン・相同性 構成要素
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / 生物種 Homo sapiens (ヒト)手法 / / 解像度 : 3.1 Å Mackinnon R / Sun J 資金援助 Organization Grant number 国 National Institutes of Health/National Heart, Lung, and Blood Institute (NIH/NHLBI) 5K99HL143037 Howard Hughes Medical Institute (HHMI)
ジャーナル : Cell / 年 : 2020タイトル : Structural Basis of Human KCNQ1 Modulation and Gating.著者 : Ji Sun / Roderick MacKinnon / 要旨 : KCNQ1, also known as Kv7.1, is a voltage-dependent K channel that regulates gastric acid secretion, salt and glucose homeostasis, and heart rhythm. Its functional properties are regulated in a tissue- ... KCNQ1, also known as Kv7.1, is a voltage-dependent K channel that regulates gastric acid secretion, salt and glucose homeostasis, and heart rhythm. Its functional properties are regulated in a tissue-specific manner through co-assembly with beta subunits KCNE1-5. In non-excitable cells, KCNQ1 forms a complex with KCNE3, which suppresses channel closure at negative membrane voltages that otherwise would close it. Pore opening is regulated by the signaling lipid PIP2. Using cryoelectron microscopy (cryo-EM), we show that KCNE3 tucks its single-membrane-spanning helix against KCNQ1, at a location that appears to lock the voltage sensor in its depolarized conformation. Without PIP2, the pore remains closed. Upon addition, PIP2 occupies a site on KCNQ1 within the inner membrane leaflet, which triggers a large conformational change that leads to dilation of the pore's gate. It is likely that this mechanism of PIP2 activation is conserved among Kv7 channels. 履歴 登録 2019年11月16日 - ヘッダ(付随情報) 公開 2019年11月27日 - マップ公開 2019年12月4日 - 更新 2024年3月6日 - 現状 2024年3月6日 処理サイト : RCSB / 状態 : 公開
すべて表示 表示を減らす
 データを開く
データを開く 基本情報
基本情報 マップデータ
マップデータ 試料
試料 キーワード
キーワード 機能・相同性情報
機能・相同性情報 Homo sapiens (ヒト)
Homo sapiens (ヒト) データ登録者
データ登録者 米国, 2件
米国, 2件  引用
引用 ジャーナル: Cell / 年: 2020
ジャーナル: Cell / 年: 2020
 構造の表示
構造の表示 ムービービューア
ムービービューア SurfView
SurfView Molmil
Molmil Jmol/JSmol
Jmol/JSmol ダウンロードとリンク
ダウンロードとリンク emd_20965.map.gz
emd_20965.map.gz EMDBマップデータ形式
EMDBマップデータ形式 emd-20965-v30.xml
emd-20965-v30.xml emd-20965.xml
emd-20965.xml EMDBヘッダ
EMDBヘッダ emd_20965.png
emd_20965.png emd-20965.cif.gz
emd-20965.cif.gz http://ftp.pdbj.org/pub/emdb/structures/EMD-20965
http://ftp.pdbj.org/pub/emdb/structures/EMD-20965 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20965
ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20965 emd_20965_validation.pdf.gz
emd_20965_validation.pdf.gz EMDB検証レポート
EMDB検証レポート emd_20965_full_validation.pdf.gz
emd_20965_full_validation.pdf.gz emd_20965_validation.xml.gz
emd_20965_validation.xml.gz emd_20965_validation.cif.gz
emd_20965_validation.cif.gz https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20965
https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20965 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20965
ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20965 リンク
リンク EMDB (EBI/PDBe) /
EMDB (EBI/PDBe) /  EMDataResource
EMDataResource マップ
マップ ダウンロード / ファイル: emd_20965.map.gz / 形式: CCP4 / 大きさ: 103 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES)
ダウンロード / ファイル: emd_20965.map.gz / 形式: CCP4 / 大きさ: 103 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) 試料の構成要素
試料の構成要素 Homo sapiens (ヒト)
Homo sapiens (ヒト) Homo sapiens (ヒト)
Homo sapiens (ヒト) Homo sapiens (ヒト)
Homo sapiens (ヒト) Homo sapiens (ヒト)
Homo sapiens (ヒト) Homo sapiens (ヒト)
Homo sapiens (ヒト) 解析
解析 試料調製
試料調製 電子顕微鏡法
電子顕微鏡法 FIELD EMISSION GUN
FIELD EMISSION GUN
 画像解析
画像解析
 ムービー
ムービー コントローラー
コントローラー



































 X (Sec.)
X (Sec.) Y (Row.)
Y (Row.) Z (Col.)
Z (Col.)