[English] 日本語
 Yorodumi
Yorodumi- EMDB-19978: Outward-open structure of Drosophila dopamine transporter bound t... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Outward-open structure of Drosophila dopamine transporter bound to an atypical non-competitive inhibitor | ||||||||||||||||||||||||||||||||||||
 Map data Map data | |||||||||||||||||||||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||||||||||||||||||||
 Keywords Keywords | SLC6A3 / Dopamine transporter / atypical inhibitors / neurotransmitter sodium symporters / MEMBRANE PROTEIN | ||||||||||||||||||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology information: / SLC-mediated transport of neurotransmitters / circadian sleep/wake cycle / response to odorant / cocaine binding / norepinephrine transport / dopamine:sodium symporter activity / regulation of presynaptic cytosolic calcium ion concentration / dopamine transport / sleep ...: / SLC-mediated transport of neurotransmitters / circadian sleep/wake cycle / response to odorant / cocaine binding / norepinephrine transport / dopamine:sodium symporter activity / regulation of presynaptic cytosolic calcium ion concentration / dopamine transport / sleep / neuronal cell body membrane / dopamine uptake involved in synaptic transmission / amino acid transport / sodium ion transmembrane transport / adult locomotory behavior / presynaptic membrane / axon / metal ion binding / plasma membrane Similarity search - Function | ||||||||||||||||||||||||||||||||||||
| Biological species |   | ||||||||||||||||||||||||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.2 Å | ||||||||||||||||||||||||||||||||||||
 Authors Authors | Pedersen CN / Yang F / Ita S / Xu Y / Akunuri R / Trampari S / Neumann CMT / Desdorf LM / Schioett B / Salvino JM ...Pedersen CN / Yang F / Ita S / Xu Y / Akunuri R / Trampari S / Neumann CMT / Desdorf LM / Schioett B / Salvino JM / Mortensen OV / Nissen P / Shahsavar A | ||||||||||||||||||||||||||||||||||||
| Funding support |  Denmark, Denmark,  United States, 11 items United States, 11 items
| ||||||||||||||||||||||||||||||||||||
 Citation Citation |  Journal: J Neurochem / Year: 2024 Journal: J Neurochem / Year: 2024Title: Cryo-EM structure of the dopamine transporter with a novel atypical non-competitive inhibitor bound to the orthosteric site. Authors: Clara Nautrup Pedersen / Fuyu Yang / Samantha Ita / Yibin Xu / Ravikumar Akunuri / Sofia Trampari / Caroline Marie Teresa Neumann / Lasse Messell Desdorf / Birgit Schiøtt / Joseph M Salvino ...Authors: Clara Nautrup Pedersen / Fuyu Yang / Samantha Ita / Yibin Xu / Ravikumar Akunuri / Sofia Trampari / Caroline Marie Teresa Neumann / Lasse Messell Desdorf / Birgit Schiøtt / Joseph M Salvino / Ole Valente Mortensen / Poul Nissen / Azadeh Shahsavar /   Abstract: The regulation of dopamine (DA) removal from the synaptic cleft is a crucial process in neurotransmission and is facilitated by the sodium- and chloride-coupled dopamine transporter DAT. ...The regulation of dopamine (DA) removal from the synaptic cleft is a crucial process in neurotransmission and is facilitated by the sodium- and chloride-coupled dopamine transporter DAT. Psychostimulant drugs, cocaine, and amphetamine, both block the uptake of DA, while amphetamine also triggers the release of DA. As a result, they prolong or even amplify neurotransmitter signaling. Atypical inhibitors of DAT lack cocaine-like rewarding effects and offer a promising strategy for the treatment of drug use disorders. Here, we present the 3.2 Å resolution cryo-electron microscopy structure of the Drosophila melanogaster dopamine transporter (dDAT) in complex with the atypical non-competitive inhibitor AC-4-248. The inhibitor partially binds at the central binding site, extending into the extracellular vestibule, and locks the transporter in an outward open conformation. Our findings propose mechanisms for the non-competitive inhibition of DAT and attenuation of cocaine potency by AC-4-248 and provide a basis for the rational design of more efficacious atypical inhibitors. | ||||||||||||||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_19978.map.gz emd_19978.map.gz | 57.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-19978-v30.xml emd-19978-v30.xml emd-19978.xml emd-19978.xml | 24.9 KB 24.9 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_19978.png emd_19978.png | 53 KB | ||
| Filedesc metadata |  emd-19978.cif.gz emd-19978.cif.gz | 7.5 KB | ||
| Others |  emd_19978_half_map_1.map.gz emd_19978_half_map_1.map.gz emd_19978_half_map_2.map.gz emd_19978_half_map_2.map.gz | 59.4 MB 59.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-19978 http://ftp.pdbj.org/pub/emdb/structures/EMD-19978 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-19978 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-19978 | HTTPS FTP |
-Related structure data
| Related structure data |  9euoMC  9eupC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_19978.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_19978.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.88963 Å | ||||||||||||||||||||||||||||||||||||
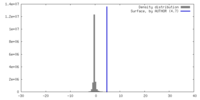
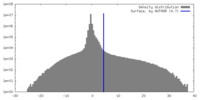
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Half map A
| File | emd_19978_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map B
| File | emd_19978_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : Drosophila melanogaster dopamine transporter bound to atypical in...
+Supramolecule #1: Drosophila melanogaster dopamine transporter bound to atypical in...
+Macromolecule #1: Sodium-dependent dopamine transporter
+Macromolecule #2: 9D5 ANTIBODY, LIGHT CHAIN
+Macromolecule #3: 9D5 ANTIBODY, HEAVY CHAIN
+Macromolecule #4: N-[3-(6-chloranyl-1,3,4,9-tetrahydropyrido[3,4-b]indol-2-yl)propy...
+Macromolecule #5: CHOLESTEROL HEMISUCCINATE
+Macromolecule #6: TRIS-HYDROXYMETHYL-METHYL-AMMONIUM
+Macromolecule #7: CHOLESTEROL
+Macromolecule #8: SODIUM ION
+Macromolecule #9: CHLORIDE ION
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 2.0 mg/mL | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| |||||||||||||||
| Grid | Model: C-flat-1.2/1.3 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY ARRAY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Atmosphere: OTHER | |||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Detector mode: COUNTING / Number grids imaged: 1 / Number real images: 14624 / Average electron dose: 61.3 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 130000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: PDB ENTRY PDB model - PDB ID: |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.2 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: cryoSPARC / Number images used: 147413 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller






 X (Sec.)
X (Sec.) Y (Row.)
Y (Row.) Z (Col.)
Z (Col.)
































 Homo sapiens (human)
Homo sapiens (human)