+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Baseplate of bacteriophage JBD30 computed in C3 symmetry | |||||||||
 Map data Map data | main map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | bacteriophage JBD30 / virion / baseplate / distal tail protein / baseplate hub / baseplate upper protein / VIRUS | |||||||||
| Function / homology | Baseplate hub protein, N-terminal attachment domain / Bacteriophage phiJL001, Gp84 / Bacteriophage phiJL001, Gp84, C-terminal / Phage conserved hypothetical protein BR0599 / Bacteriophage phiJL001 Gp84 C-terminal domain-containing protein / Uncharacterized protein / Virion structural protein Function and homology information Function and homology information | |||||||||
| Biological species |  Pseudomonas phage JBD30 (virus) Pseudomonas phage JBD30 (virus) | |||||||||
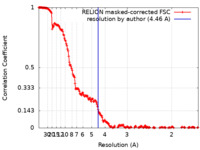
| Method | single particle reconstruction / cryo EM / Resolution: 4.46 Å | |||||||||
 Authors Authors | Valentova L / Fuzik T / Plevka P | |||||||||
| Funding support |  Czech Republic, European Union, 2 items Czech Republic, European Union, 2 items
| |||||||||
 Citation Citation |  Journal: EMBO J / Year: 2024 Journal: EMBO J / Year: 2024Title: Structure and replication of Pseudomonas aeruginosa phage JBD30. Authors: Lucie Valentová / Tibor Füzik / Jiří Nováček / Zuzana Hlavenková / Jakub Pospíšil / Pavel Plevka /  Abstract: Bacteriophages are the most abundant biological entities on Earth, but our understanding of many aspects of their lifecycles is still incomplete. Here, we have structurally analysed the infection ...Bacteriophages are the most abundant biological entities on Earth, but our understanding of many aspects of their lifecycles is still incomplete. Here, we have structurally analysed the infection cycle of the siphophage Casadabanvirus JBD30. Using its baseplate, JBD30 attaches to Pseudomonas aeruginosa via the bacterial type IV pilus, whose subsequent retraction brings the phage to the bacterial cell surface. Cryo-electron microscopy structures of the baseplate-pilus complex show that the tripod of baseplate receptor-binding proteins attaches to the outer bacterial membrane. The tripod and baseplate then open to release three copies of the tape-measure protein, an event that is followed by DNA ejection. JBD30 major capsid proteins assemble into procapsids, which expand by 7% in diameter upon filling with phage dsDNA. The DNA-filled heads are finally joined with 180-nm-long tails, which bend easily because flexible loops mediate contacts between the successive discs of major tail proteins. It is likely that the structural features and replication mechanisms described here are conserved among siphophages that utilize the type IV pili for initial cell attachment. #1:  Journal: Embo J. / Year: 2024 Journal: Embo J. / Year: 2024Title: Structure and replication of Pseudomonas aeruginosa phage JBD30 Authors: Valentova L / Plevka P / Fuzik T / Novacek J / Pospisil J | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_19263.map.gz emd_19263.map.gz | 54.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-19263-v30.xml emd-19263-v30.xml emd-19263.xml emd-19263.xml | 23.7 KB 23.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_19263_fsc.xml emd_19263_fsc.xml | 19.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_19263.png emd_19263.png | 143.7 KB | ||
| Masks |  emd_19263_msk_1.map emd_19263_msk_1.map | 600.7 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-19263.cif.gz emd-19263.cif.gz | 7.3 KB | ||
| Others |  emd_19263_half_map_1.map.gz emd_19263_half_map_1.map.gz emd_19263_half_map_2.map.gz emd_19263_half_map_2.map.gz | 486.6 MB 486.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-19263 http://ftp.pdbj.org/pub/emdb/structures/EMD-19263 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-19263 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-19263 | HTTPS FTP |
-Related structure data
| Related structure data |  8rk7MC  8rk3C  8rk4C  8rk5C  8rk6C  8rk8C  8rk9C  8rkaC  8rkbC  8rkcC  8rknC  8rkoC  8rkxC  8rqeC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_19263.map.gz / Format: CCP4 / Size: 600.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_19263.map.gz / Format: CCP4 / Size: 600.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | main map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.8336 Å | ||||||||||||||||||||||||||||||||||||
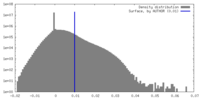
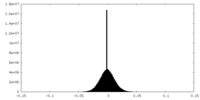
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_19263_msk_1.map emd_19263_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
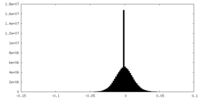
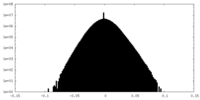
| Density Histograms |
-Half map: half map 2
| File | emd_19263_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half_map_2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
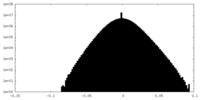
| Density Histograms |
-Half map: half map 1
| File | emd_19263_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half_map_1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Pseudomonas phage JBD30
| Entire | Name:  Pseudomonas phage JBD30 (virus) Pseudomonas phage JBD30 (virus) |
|---|---|
| Components |
|
-Supramolecule #1: Pseudomonas phage JBD30
| Supramolecule | Name: Pseudomonas phage JBD30 / type: virus / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 Details: Phage JBD30 was propagated in P. aeruginosa strain BAA-28 and purified using CsCl gradient. NCBI-ID: 1223260 / Sci species name: Pseudomonas phage JBD30 / Virus type: VIRION / Virus isolate: STRAIN / Virus enveloped: No / Virus empty: No |
|---|---|
| Host (natural) | Organism:  |
| Molecular weight | Theoretical: 516 KDa |
| Virus shell | Shell ID: 1 / Name: JBD30 capsid / Diameter: 640.0 Å / T number (triangulation number): 7 |
-Macromolecule #1: DUF2163 domain-containing protein
| Macromolecule | Name: DUF2163 domain-containing protein / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Pseudomonas phage JBD30 (virus) Pseudomonas phage JBD30 (virus) |
| Molecular weight | Theoretical: 29.979053 KDa |
| Sequence | String: MSFNSRESSL ADGQPVRLYQ FSRGAIRWSY NSSDRDITYQ NQIFRTVPGG ITDNGIICSG DPQSDQFVIT APADLDVALL YKTRSPSDA IDLVVYDMHY GDAEAAVSWV GQIGDVDWPT VDSCRITCVS EDELMDQPGL TDTYCRTCTA IVGDHRCKVN L VPYRVTLT ...String: MSFNSRESSL ADGQPVRLYQ FSRGAIRWSY NSSDRDITYQ NQIFRTVPGG ITDNGIICSG DPQSDQFVIT APADLDVALL YKTRSPSDA IDLVVYDMHY GDAEAAVSWV GQIGDVDWPT VDSCRITCVS EDELMDQPGL TDTYCRTCTA IVGDHRCKVN L VPYRVTLT PQSVSAWVIS SGVVAGYADG WFTGGYVEWQ VDGDNYDRRF IEQHAGADLH ILGGTEGIPA GGQLRVYPGC DG LAQTCDD KFSNLPNFRG FNAMQGKSPF DGDQVW UniProtKB: Bacteriophage phiJL001 Gp84 C-terminal domain-containing protein |
-Macromolecule #2: Virion structural protein
| Macromolecule | Name: Virion structural protein / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Pseudomonas phage JBD30 (virus) Pseudomonas phage JBD30 (virus) |
| Molecular weight | Theoretical: 62.163148 KDa |
| Sequence | String: MATFPGFQVP KPVEGIVAGI TPNIDALELN QDISLAAVAA STWGGAYGAH QPVEVIHSTY QAVHQSALEE NYYNRLWLIP TAMELGNVV STQIRPASVW NAYFSPRTLT AIDREAADGI TLSGQASPPL GFAALEERTW TVSIGTDGPP VVNARIVWRL Q GEPNLVLV ...String: MATFPGFQVP KPVEGIVAGI TPNIDALELN QDISLAAVAA STWGGAYGAH QPVEVIHSTY QAVHQSALEE NYYNRLWLIP TAMELGNVV STQIRPASVW NAYFSPRTLT AIDREAADGI TLSGQASPPL GFAALEERTW TVSIGTDGPP VVNARIVWRL Q GEPNLVLV ITGNRIIAWT FAPDWGDSIV ERLSASTNIL QSESAVTQRR AMRLAPRREF DANMYAVDRE RQLLDMTLFG WG ARIWALP IWPDIQLLHQ PLAAGSLGIP CDTAGLDFRD GGLAMLRGED AFTYEVVEVK TVTASGLDLV RPVQAAWGTG SRL YPVRTA QLTEQPTLTR LTDTAQSARV SFLVMEPSAW PELMPATTYR GRPVLEQRPD ESEDLTSSYQ RLLSTLDNGS AIPR VTDVA GMALPVIGHR WIGMGRAERS AFRSLVYALR GQQKPLWVPT HADDLTLVAT VSQLSTALDV RNIGYARFAN GRPGR RDIR IELYDGTVYH RRILTSTELD ADTERVAIDA ALGRLVEPTD VARICFMALC SAASDVVEIE HVTDSEGVAT AALTFK GVR DDEF UniProtKB: Virion structural protein |
-Macromolecule #3: Tip attachment protein J domain-containing protein
| Macromolecule | Name: Tip attachment protein J domain-containing protein / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Pseudomonas phage JBD30 (virus) Pseudomonas phage JBD30 (virus) |
| Molecular weight | Theoretical: 79.898781 KDa |
| Sequence | String: MGAKPKAQTV GWRYYFDIHF ALGKKVDEVC AIRASGKTAW KGSITSNGQV RINAPELFGG DKGEGGLDGT LDVLFGEEDQ GVLPRLAAM LGGLVPAFRG VTTCFYSGLV TSVNPYPKKW EILRRGGNRL WDGNPWYPEK QFVWLADGQI KAMNPAHILY L VYTGRDFR ...String: MGAKPKAQTV GWRYYFDIHF ALGKKVDEVC AIRASGKTAW KGSITSNGQV RINAPELFGG DKGEGGLDGT LDVLFGEEDQ GVLPRLAAM LGGLVPAFRG VTTCFYSGLV TSVNPYPKKW EILRRGGNRL WDGNPWYPEK QFVWLADGQI KAMNPAHILY L VYTGRDFR GLARTRMDEA SWRAAADTLY AEGFGLCFEW TRSDSFKNFC ETVKSHIGAE VYPNRQTGQI SIRLLRDDYN VA DLPLFDE DSGLLEITQE KTGSTSLAPS QLIVKYIDQI DGAQRQIIVN NNAVAASQGR RSSEEIEFLG VPTGELAGRV GER EMRLKT TGLKRYKGVF DRRARSLNPG QPFLIRSTPR GIPETVVRVG RIEDNFLGDG KITLTVVQDQ FNLPATTGVA PPPP GWTPP DRTPRAITVR RLIEAPYREL AGVIDPANLQ LLDVSASYLA ALAEAPTSLS QSYTLTDRVG SSGAFVDRGT GDWCP TGLL AAELPLAAGP NVVTLTNATR LEDVTVGQAA VVDDEIVRVD AVNYASGTVT LARGCADTVP AKHLAGARVW FYDTFE AVD ETVYSQGVTL QARLLTNTSE GQLAPALAAT DSLTLTGRQG KPYPPGQFRI NGSAYPTKVY GALSVSWAKR DRIGQAD QL IDTTVGNIGP EDGATVTLQV YSGTTLKRTY AGLTSSSWSY PLAEDMADGP LQDVRLVLRS VRDGIDSWQQ HDITIERH G LGFRLGEELG GVSA UniProtKB: Uncharacterized protein |
-Macromolecule #4: FE (III) ION
| Macromolecule | Name: FE (III) ION / type: ligand / ID: 4 / Number of copies: 1 / Formula: FE |
|---|---|
| Molecular weight | Theoretical: 55.845 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 Component:
Details: 10 mM NaCl, 10 mM MgSO4, 50 mM Tris-HCl | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 15 sec. / Pretreatment - Atmosphere: OTHER / Details: Gatan Solarus II | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277.15 K / Instrument: FEI VITROBOT MARK IV Details: blotting force 0, blotting time 2s, waiting time 15s. | ||||||||||||
| Details | phage titer 10^11 PFU/ml |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 10 eV |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Digitization - Dimensions - Width: 5760 pixel / Digitization - Dimensions - Height: 4092 pixel / Number grids imaged: 1 / Number real images: 12356 / Average exposure time: 2.0 sec. / Average electron dose: 34.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 1.6 µm / Nominal defocus min: 0.6 µm / Nominal magnification: 105000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: OTHER |
|---|---|
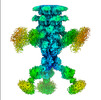
| Output model |  PDB-8rk7: |
 Movie
Movie Controller
Controller


























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)