+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
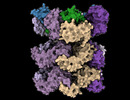
| Title | Structure of the 55LCC ATPase complex | ||||||||||||
 Map data Map data | Sharpened lid and ATPase composite map (after local refinement) | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | DNA replication / AAA+ ATPases / proteostasis / DNA BINDING PROTEIN | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationpreribosome binding / mitotic spindle disassembly / VCP-NPL4-UFD1 AAA ATPase complex / retrograde protein transport, ER to cytosol / non-chaperonin molecular chaperone ATPase / polyubiquitin modification-dependent protein binding / autophagosome maturation / ribosomal large subunit biogenesis / brain development / spindle ...preribosome binding / mitotic spindle disassembly / VCP-NPL4-UFD1 AAA ATPase complex / retrograde protein transport, ER to cytosol / non-chaperonin molecular chaperone ATPase / polyubiquitin modification-dependent protein binding / autophagosome maturation / ribosomal large subunit biogenesis / brain development / spindle / spermatogenesis / proteasome-mediated ubiquitin-dependent protein catabolic process / cell differentiation / DNA replication / cell division / DNA repair / ATP hydrolysis activity / ATP binding / identical protein binding / nucleus / cytosol / cytoplasm Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.5 Å | ||||||||||||
 Authors Authors | Foglizzo M / Degtjarik O / Zeqiraj E | ||||||||||||
| Funding support |  United Kingdom, 3 items United Kingdom, 3 items
| ||||||||||||
 Citation Citation |  Journal: Cell / Year: 2024 Journal: Cell / Year: 2024Title: The SPATA5-SPATA5L1 ATPase complex directs replisome proteostasis to ensure genome integrity. Authors: Vidhya Krishnamoorthy / Martina Foglizzo / Robert L Dilley / Angela Wu / Arindam Datta / Parul Dutta / Lisa J Campbell / Oksana Degtjarik / Laura J Musgrove / Antonio N Calabrese / Elton ...Authors: Vidhya Krishnamoorthy / Martina Foglizzo / Robert L Dilley / Angela Wu / Arindam Datta / Parul Dutta / Lisa J Campbell / Oksana Degtjarik / Laura J Musgrove / Antonio N Calabrese / Elton Zeqiraj / Roger A Greenberg /   Abstract: Ubiquitin-dependent unfolding of the CMG helicase by VCP/p97 is required to terminate DNA replication. Other replisome components are not processed in the same fashion, suggesting that additional ...Ubiquitin-dependent unfolding of the CMG helicase by VCP/p97 is required to terminate DNA replication. Other replisome components are not processed in the same fashion, suggesting that additional mechanisms underlie replication protein turnover. Here, we identify replisome factor interactions with a protein complex composed of AAA+ ATPases SPATA5-SPATA5L1 together with heterodimeric partners C1orf109-CINP (55LCC). An integrative structural biology approach revealed a molecular architecture of SPATA5-SPATA5L1 N-terminal domains interacting with C1orf109-CINP to form a funnel-like structure above a cylindrically shaped ATPase motor. Deficiency in the 55LCC complex elicited ubiquitin-independent proteotoxicity, replication stress, and severe chromosome instability. 55LCC showed ATPase activity that was specifically enhanced by replication fork DNA and was coupled to cysteine protease-dependent cleavage of replisome substrates in response to replication fork damage. These findings define 55LCC-mediated proteostasis as critical for replication fork progression and genome stability and provide a rationale for pathogenic variants seen in associated human neurodevelopmental disorders. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_19177.map.gz emd_19177.map.gz | 196.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-19177-v30.xml emd-19177-v30.xml emd-19177.xml emd-19177.xml | 33.4 KB 33.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_19177_fsc.xml emd_19177_fsc.xml | 12.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_19177.png emd_19177.png | 122.2 KB | ||
| Masks |  emd_19177_msk_1.map emd_19177_msk_1.map | 209.3 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-19177.cif.gz emd-19177.cif.gz | 8.2 KB | ||
| Others |  emd_19177_additional_1.map.gz emd_19177_additional_1.map.gz emd_19177_additional_2.map.gz emd_19177_additional_2.map.gz emd_19177_additional_3.map.gz emd_19177_additional_3.map.gz emd_19177_additional_4.map.gz emd_19177_additional_4.map.gz emd_19177_half_map_1.map.gz emd_19177_half_map_1.map.gz emd_19177_half_map_2.map.gz emd_19177_half_map_2.map.gz | 100.7 MB 102 MB 197.1 MB 100.4 MB 194.1 MB 194.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-19177 http://ftp.pdbj.org/pub/emdb/structures/EMD-19177 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-19177 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-19177 | HTTPS FTP |
-Related structure data
| Related structure data |  8rhnMC  8cihC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_19177.map.gz / Format: CCP4 / Size: 209.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_19177.map.gz / Format: CCP4 / Size: 209.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened lid and ATPase composite map (after local refinement) | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.74 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_19177_msk_1.map emd_19177_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Unsharpened ATPase map (after local refinement)
| File | emd_19177_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unsharpened ATPase map (after local refinement) | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Unsharpened lid map (after local refinement)
| File | emd_19177_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unsharpened lid map (after local refinement) | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Sharpened full map (before local refinement)
| File | emd_19177_additional_3.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened full map (before local refinement) | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Unsharpened full map (before local refinement)
| File | emd_19177_additional_4.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unsharpened full map (before local refinement) | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map A
| File | emd_19177_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map B
| File | emd_19177_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Structure of the 55LCC ATPase complex
| Entire | Name: Structure of the 55LCC ATPase complex |
|---|---|
| Components |
|
-Supramolecule #1: Structure of the 55LCC ATPase complex
| Supramolecule | Name: Structure of the 55LCC ATPase complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 649 KDa |
-Macromolecule #1: ATPase family gene 2 protein homolog A
| Macromolecule | Name: ATPase family gene 2 protein homolog A / type: protein_or_peptide / ID: 1 / Number of copies: 8 / Enantiomer: LEVO / EC number: non-chaperonin molecular chaperone ATPase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 101.307883 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSYYHHHHHH DYDIPTTENL YFQGAMGMSS KKNRKRLNQS AENGSSLPSA ASSCAEARAP SAGSDFAATS GTLTVTNLLE KVDDKIPKT FQNSLIHLGL NTMKSANICI GRPVLLTSLN GKQEVYTAWP MAGFPGGKVG LSEMAQKNVG VRPGDAIQVQ P LVGAVLQA ...String: MSYYHHHHHH DYDIPTTENL YFQGAMGMSS KKNRKRLNQS AENGSSLPSA ASSCAEARAP SAGSDFAATS GTLTVTNLLE KVDDKIPKT FQNSLIHLGL NTMKSANICI GRPVLLTSLN GKQEVYTAWP MAGFPGGKVG LSEMAQKNVG VRPGDAIQVQ P LVGAVLQA EEMDVALSDK DMEINEEELT GCILRKLDGK IVLPGNFLYC TFYGRPYKLQ VLRVKGADGM ILGGPQSDSD TD AQRMAFE QSSMETSSLE LSLQLSQLDL EDTQIPTSRS TPYKPIDDRI TNKASDVLLD VTQSPGDGSG LMLEEVTGLK CNF ESAREG NEQLTEEERL LKFSIGAKCN TDTFYFISST TRVNFTEIDK NSKEQDNQFK VTYDMIGGLS SQLKAIREII ELPL KQPEL FKSYGIPAPR GVLLYGPPGT GKTMIARAVA NEVGAYVSVI NGPEIISKFY GETEAKLRQI FAEATLRHPS IIFID ELDA LCPKREGAQN EVEKRVVASL LTLMDGIGSE VSEGQVLVLG ATNRPHALDA ALRRPGRFDK EIEIGVPNAQ DRLDIL QKL LRRVPHLLTE AELLQLANSA HGYVGADLKV LCNEAGLCAL RRILKKQPNL PDVKVAGLVK ITLKDFLQAM NDIRPSA MR EIAIDVPNVS WSDIGGLESI KLKLEQAVEW PLKHPESFIR MGIQPPKGVL LYGPPGCSKT MIAKALANES GLNFLAIK G PELMNKYVGE SERAVRETFR KARAVAPSII FFDELDALAV ERGSSLGAGN VADRVLAQLL TEMDGIEQLK DVTILAATN RPDRIDKALM RPGRIDRIIY VPLPDAATRR EIFKLQFHSM PVSNEVDLDE LILQTDAYSG AEIVAVCREA ALLALEEDIQ ANLIMKRHF TQALSTVTPR IPESLRRFYE DYQEKSGLHT L UniProtKB: ATPase family gene 2 protein homolog A |
-Macromolecule #2: ATPase family gene 2 protein homolog B
| Macromolecule | Name: ATPase family gene 2 protein homolog B / type: protein_or_peptide / ID: 2 / Number of copies: 4 / Enantiomer: LEVO / EC number: non-chaperonin molecular chaperone ATPase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 83.362836 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MDYKDDDDKG GGSENLYFQG AGSTMAPDSD PFPEGPLLKL LPLDARDRGT QRCRLGPAAL HALGARLGSA VKISLPDGGS CLCTAWPRR DGADGFVQLD PLCASPGAAV GASRSRRSLS LNRLLLVPCP PLRRVAVWPV LRERAGAPGA RNTAAVLEAA Q ELLRNRPI ...String: MDYKDDDDKG GGSENLYFQG AGSTMAPDSD PFPEGPLLKL LPLDARDRGT QRCRLGPAAL HALGARLGSA VKISLPDGGS CLCTAWPRR DGADGFVQLD PLCASPGAAV GASRSRRSLS LNRLLLVPCP PLRRVAVWPV LRERAGAPGA RNTAAVLEAA Q ELLRNRPI SLGHVVVAPP GAPGLVAALH IVGGTPSPDP AGLVTPRTRV SLGGEPPSEA QPQPEVPLGG LSEAADSLRE LL RLPLRYP RALTALGLAV PRGVLLAGPP GVGKTQLVRA VAREAGAELL AVSAPALQGS RPGETEENVR RVFQRARELA SRG PSLLFL DEMDALCPQR GSRAPESRVV AQVLTLLDGA SGDREVVVVG ATNRPDALDP ALRRPGRFDR EVVIGTPTLK QRKE ILQVI TSKMPISSHV DLGLLAEMTV GYVGADLTAL CREAAMHALL HSEKNQDNPV IDEIDFLEAF KNIQPSSFRS VIGLM DIKP VDWEEIGGLE DVKLKLKQSI EWPLKFPWEF VRMGLTQPKG VLLYGPPGCA KTTLVRALAT SCHCSFVSVS GADLFS PFV GDSEKVLSQI FRQARASTPA ILFLDEIDSI LGARSASKTG CDVQERVLSV LLNELDGVGL KTIERRGSKS SQQEFQE VF NRSVMIIAAT NRPDVLDTAL LRPGRLDKII YIPPPDHKGR LSILKVCTKT MPIGPDVSLE NLAAETCFFS GADLRNLC T EAALLALQEN GLDATTVKQE HFLKSLKTVK PSLSCKDLAL YENLFKKEGF SNVEGI UniProtKB: ATPase family gene 2 protein homolog B |
-Macromolecule #3: cDNA FLJ55172
| Macromolecule | Name: cDNA FLJ55172 / type: protein_or_peptide / ID: 3 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 29.439279 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSAWSHPQFE KGGGSGGGSG GSAWSHPQFE KGAGSENLYF QGAGSDSLEF IASKLAGGGS TMTQDRPLLA VQEALKKCFP VVEEQQGLW QSALRDCQPL LSSLSNLAEQ LQAAQNLRFE DVPALRAFPD LKERLRRKQL VAGDIVLDKL GERLAILLKV R DMVSSHVE ...String: MSAWSHPQFE KGGGSGGGSG GSAWSHPQFE KGAGSENLYF QGAGSDSLEF IASKLAGGGS TMTQDRPLLA VQEALKKCFP VVEEQQGLW QSALRDCQPL LSSLSNLAEQ LQAAQNLRFE DVPALRAFPD LKERLRRKQL VAGDIVLDKL GERLAILLKV R DMVSSHVE RVFQIYEQHA DTVGIDAVLQ PSAVSPSVAD MLEWLQDIER HYRKSYLKRK YLLSSIQWGD LANIQALPKA WD RISKDEH QDLVQDILLN VSFFLEE UniProtKB: cDNA FLJ55172 |
-Macromolecule #4: Cyclin-dependent kinase 2-interacting protein
| Macromolecule | Name: Cyclin-dependent kinase 2-interacting protein / type: protein_or_peptide / ID: 4 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 27.462139 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSYYHHHHHH DYDIPTTENL YFQGAMEAKT LGTVTPRKPV LSVSARKIKD NAADWHNLIL KWETLNDAGF TTANNIANLK ISLLNKDKI ELDSSSPASK ENEEKVCLEY NEELEKLCEE LQATLDGLTK IQVKMEKLSS TTKGICELEN YHYGEESKRP P LFHTWPTT ...String: MSYYHHHHHH DYDIPTTENL YFQGAMEAKT LGTVTPRKPV LSVSARKIKD NAADWHNLIL KWETLNDAGF TTANNIANLK ISLLNKDKI ELDSSSPASK ENEEKVCLEY NEELEKLCEE LQATLDGLTK IQVKMEKLSS TTKGICELEN YHYGEESKRP P LFHTWPTT HFYEVSHKLL EMYRKELLLK RTVAKELAHT GDPDLTLSYL SMWLHQPYVE SDSRLHLESM LLETGHRAL UniProtKB: Cyclin-dependent kinase 2-interacting protein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 3.7 mg/mL | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
Details: 25 mM Tris pH 7.5, 400 mM NaCl, 5% (v/v) glycerol, 1 mM EGTA, 2 mM ADP, 2 mM MgCl2 and 1 mM TCEP | |||||||||||||||||||||
| Grid | Model: Quantifoil R3.5/1 / Material: COPPER / Mesh: 200 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 0.00038 kPa Details: Quantifoil R3.5/1 200-mesh grids (Quantifoil Micro Tools GmbH) were glow-discharged for 30 s at 12 mA and 0.38 mBar pressure using a PELCO easiGlow system (Ted Pella). | |||||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV / Details: blot force = 0 N blot time = 8 s. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Phase plate: VOLTA PHASE PLATE / Energy filter - Name: TFS Selectris / Energy filter - Slit width: 10 eV |
| Image recording | Film or detector model: TFS FALCON 4i (4k x 4k) / Number grids imaged: 1 / Number real images: 27595 / Average exposure time: 2.99 sec. / Average electron dose: 37.6 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 3.1 µm / Nominal defocus min: 1.2 µm / Nominal magnification: 165000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | Chain - Source name: AlphaFold / Chain - Initial model type: in silico model |
|---|---|
| Refinement | Space: REAL / Protocol: RIGID BODY FIT / Overall B value: 127.55 |
| Output model |  PDB-8rhn: |
 Movie
Movie Controller
Controller







 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)