[English] 日本語
 Yorodumi
Yorodumi- EMDB-18310: Helical reconstruction of yeast eisosome protein Pil1 bound to me... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Helical reconstruction of yeast eisosome protein Pil1 bound to membrane composed of lipid mixture +PIP2/+sterol (DOPC, DOPE, DOPS, cholesterol, PI(4,5)P2 35:20:20:15:10) | |||||||||||||||
 Map data Map data | Sharpened helical map A&C, non-uniform refinement (D1, rise=5.408, twist=133.595) | |||||||||||||||
 Sample Sample |
| |||||||||||||||
 Keywords Keywords | BAR domain / lipid reconstitution / membrane microdomain / LIPID BINDING PROTEIN | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationprotein localization to eisosome filament / eisosome filament / eisosome assembly / eisosome / lipid droplet / cell periphery / endocytosis / intracellular protein localization / mitochondrial outer membrane / lipid binding ...protein localization to eisosome filament / eisosome filament / eisosome assembly / eisosome / lipid droplet / cell periphery / endocytosis / intracellular protein localization / mitochondrial outer membrane / lipid binding / mitochondrion / plasma membrane / cytoplasm Similarity search - Function | |||||||||||||||
| Biological species |  | |||||||||||||||
| Method | helical reconstruction / cryo EM / Resolution: 3.61 Å | |||||||||||||||
 Authors Authors | Kefauver JM / Zou L / Desfosses A / Loewith RJ | |||||||||||||||
| Funding support | European Union,  Switzerland, 4 items Switzerland, 4 items
| |||||||||||||||
 Citation Citation | Journal: Acta Crystallogr D Struct Biol / Year: 2018 Title: Real-space refinement in PHENIX for cryo-EM and crystallography. Authors: Pavel V Afonine / Billy K Poon / Randy J Read / Oleg V Sobolev / Thomas C Terwilliger / Alexandre Urzhumtsev / Paul D Adams /    Abstract: This article describes the implementation of real-space refinement in the phenix.real_space_refine program from the PHENIX suite. The use of a simplified refinement target function enables very fast ...This article describes the implementation of real-space refinement in the phenix.real_space_refine program from the PHENIX suite. The use of a simplified refinement target function enables very fast calculation, which in turn makes it possible to identify optimal data-restraint weights as part of routine refinements with little runtime cost. Refinement of atomic models against low-resolution data benefits from the inclusion of as much additional information as is available. In addition to standard restraints on covalent geometry, phenix.real_space_refine makes use of extra information such as secondary-structure and rotamer-specific restraints, as well as restraints or constraints on internal molecular symmetry. The re-refinement of 385 cryo-EM-derived models available in the Protein Data Bank at resolutions of 6 Å or better shows significant improvement of the models and of the fit of these models to the target maps. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_18310.map.gz emd_18310.map.gz | 944.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-18310-v30.xml emd-18310-v30.xml emd-18310.xml emd-18310.xml | 33.5 KB 33.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_18310.png emd_18310.png | 210.2 KB | ||
| Masks |  emd_18310_msk_1.map emd_18310_msk_1.map | 1000 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-18310.cif.gz emd-18310.cif.gz | 7.1 KB | ||
| Others |  emd_18310_additional_1.map.gz emd_18310_additional_1.map.gz emd_18310_additional_2.map.gz emd_18310_additional_2.map.gz emd_18310_additional_3.map.gz emd_18310_additional_3.map.gz emd_18310_additional_4.map.gz emd_18310_additional_4.map.gz emd_18310_additional_5.map.gz emd_18310_additional_5.map.gz emd_18310_additional_6.map.gz emd_18310_additional_6.map.gz emd_18310_additional_7.map.gz emd_18310_additional_7.map.gz emd_18310_half_map_1.map.gz emd_18310_half_map_1.map.gz emd_18310_half_map_2.map.gz emd_18310_half_map_2.map.gz | 493.8 MB 487 MB 494.6 MB 496.4 MB 496.3 MB 492.5 MB 496.3 MB 926.2 MB 926.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-18310 http://ftp.pdbj.org/pub/emdb/structures/EMD-18310 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-18310 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-18310 | HTTPS FTP |
-Related structure data
| Related structure data |  8qbdMC  8qb7C  8qb8C  8qb9C  8qbbC  8qbeC  8qbfC  8qbgC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_18310.map.gz / Format: CCP4 / Size: 1000 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_18310.map.gz / Format: CCP4 / Size: 1000 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened helical map A&C, non-uniform refinement (D1, rise=5.408, twist=133.595) | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.83 Å | ||||||||||||||||||||||||||||||||||||
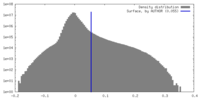
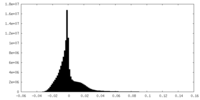
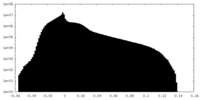
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
+Mask #1
+Additional map: Unsharpened helical map B (D3, rise=14.548, twist=276.716)
+Additional map: Unsharpened helical map K (D1, rise=5.740, twist=152.300)
+Additional map: Unsharpened helical map E (D1, rise=4.980, twist=-161.053)
+Additional map: Unsharpened helical map D (D2, rise=11.142, twist=-137.653)
+Additional map: Unsharpened helical map J (D1, rise=5.077, twist=-136.507)
+Additional map: Unsharpened helical map F&G&H (D4, rise=20.968, twist=-140.956)
+Additional map: Unsharpened helical map A&C, non-uniform refinement (D1, rise=5.408,...
+Half map: Helical map A&C, non-uniform refinement - half map B
+Half map: Helical map A&C, non-uniform refinement - half map A
- Sample components
Sample components
-Entire : Helical assembly of recombinant Pil1 protein tubulating +PIP2/+st...
| Entire | Name: Helical assembly of recombinant Pil1 protein tubulating +PIP2/+sterol lipid mixture (DOPC, DOPE, DOPS, cholesterol, PI(4,5)P2 35:20:20:15:10) |
|---|---|
| Components |
|
-Supramolecule #1: Helical assembly of recombinant Pil1 protein tubulating +PIP2/+st...
| Supramolecule | Name: Helical assembly of recombinant Pil1 protein tubulating +PIP2/+sterol lipid mixture (DOPC, DOPE, DOPS, cholesterol, PI(4,5)P2 35:20:20:15:10) type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Sphingolipid long chain base-responsive protein PIL1
| Macromolecule | Name: Sphingolipid long chain base-responsive protein PIL1 / type: protein_or_peptide / ID: 1 / Number of copies: 14 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 38.393043 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MHRTYSLRNS RAPTASQLQN PPPPPSTTKG RFFGKGGLAY SFRRSAAGAF GPELSRKLSQ LVKIEKNVLR SMELTANERR DAAKQLSIW GLENDDDVSD ITDKLGVLIY EVSELDDQFI DRYDQYRLTL KSIRDIEGSV QPSRDRKDKI TDKIAYLKYK D PQSPKIEV ...String: MHRTYSLRNS RAPTASQLQN PPPPPSTTKG RFFGKGGLAY SFRRSAAGAF GPELSRKLSQ LVKIEKNVLR SMELTANERR DAAKQLSIW GLENDDDVSD ITDKLGVLIY EVSELDDQFI DRYDQYRLTL KSIRDIEGSV QPSRDRKDKI TDKIAYLKYK D PQSPKIEV LEQELVRAEA ESLVAEAQLS NITRSKLRAA FNYQFDSIIE HSEKIALIAG YGKALLELLD DSPVTPGETR PA YDGYEAS KQIIIDAESA LNEWTLDSAQ VKPTLSFKQD YEDFEPEEGE EEEEEDGQGR WSEDEQEDGQ IEEPEQEEEG AVE EHEQVG HQQSESLPQQ TTA UniProtKB: Sphingolipid long chain base-responsive protein PIL1 |
-Macromolecule #2: D-MYO-INOSITOL-1,4,5-TRIPHOSPHATE
| Macromolecule | Name: D-MYO-INOSITOL-1,4,5-TRIPHOSPHATE / type: ligand / ID: 2 / Number of copies: 14 / Formula: I3P |
|---|---|
| Molecular weight | Theoretical: 420.096 Da |
| Chemical component information |  ChemComp-I3P: |
-Macromolecule #3: O-[(R)-{[(2R)-2,3-bis(octadecanoyloxy)propyl]oxy}(hydroxy)phospho...
| Macromolecule | Name: O-[(R)-{[(2R)-2,3-bis(octadecanoyloxy)propyl]oxy}(hydroxy)phosphoryl]-L-serine type: ligand / ID: 3 / Number of copies: 14 / Formula: P5S |
|---|---|
| Molecular weight | Theoretical: 792.075 Da |
| Chemical component information |  ChemComp-P5S: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | helical array |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 / Details: 20mM HEPES, pH 7.4, 150mM KoAc, 2mM MgAc |
|---|---|
| Grid | Model: EMS Lacey Carbon / Support film - Material: CARBON / Support film - topology: LACEY |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 90 % / Chamber temperature: 291 K / Instrument: LEICA EM GP |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 1.8 µm / Nominal defocus min: 0.6 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Applied symmetry - Helical parameters - Δz: 5.408 Å Applied symmetry - Helical parameters - Δ&Phi: 133.595 ° Applied symmetry - Helical parameters - Axial symmetry: D1 (2x1 fold dihedral) Resolution.type: BY AUTHOR / Resolution: 3.61 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 77414 |
|---|---|
| Startup model | Type of model: NONE |
| Final angle assignment | Type: NOT APPLICABLE |
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Source name: AlphaFold / Chain - Initial model type: in silico model |
|---|---|
| Refinement | Space: REAL |
| Output model |  PDB-8qbd: |
 Movie
Movie Controller
Controller










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)