登録情報 データベース : EMDB / ID : EMD-14875タイトル CryoEM structure of HSP90-CDC37-BRAF(V600E) complex. 複合体 : HSP90-CDC37-BRAF(V600E) complexタンパク質・ペプチド : Heat shock protein HSP 90-betaタンパク質・ペプチド : Hsp90 co-chaperone Cdc37タンパク質・ペプチド : Serine/threonine-protein kinase B-rafリガンド : ADENOSINE-5'-TRIPHOSPHATE / / 機能・相同性 分子機能 ドメイン・相同性 構成要素
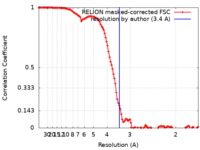
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / 生物種 Homo sapiens (ヒト)手法 / / 解像度 : 3.4 Å Oberoi J / Pearl LH 資金援助 Organization Grant number 国 Wellcome Trust
ジャーナル : Nat Commun / 年 : 2022タイトル : HSP90-CDC37-PP5 forms a structural platform for kinase dephosphorylation.著者 : Jasmeen Oberoi / Xavi Aran Guiu / Emily A Outwin / Pascale Schellenberger / Theodoros I Roumeliotis / Jyoti S Choudhary / Laurence H Pearl / 要旨 : Activation of client protein kinases by the HSP90 molecular chaperone system is affected by phosphorylation at multiple sites on HSP90, the kinase-specific co-chaperone CDC37, and the kinase client ... Activation of client protein kinases by the HSP90 molecular chaperone system is affected by phosphorylation at multiple sites on HSP90, the kinase-specific co-chaperone CDC37, and the kinase client itself. Removal of regulatory phosphorylation from client kinases and their release from the HSP90-CDC37 system depends on the Ser/Thr phosphatase PP5, which associates with HSP90 via its N-terminal TPR domain. Here, we present the cryoEM structure of the oncogenic protein kinase client BRAF bound to HSP90-CDC37, showing how the V600E mutation favours BRAF association with HSP90-CDC37. Structures of HSP90-CDC37-BRAF complexes with PP5 in autoinhibited and activated conformations, together with proteomic analysis of its phosphatase activity on BRAF and CRAF, reveal how PP5 is activated by recruitment to HSP90 complexes. PP5 comprehensively dephosphorylates client proteins, removing interaction sites for regulatory partners such as 14-3-3 proteins and thus performing a 'factory reset' of the kinase prior to release. 履歴 登録 2022年5月3日 - ヘッダ(付随情報) 公開 2022年12月14日 - マップ公開 2022年12月14日 - 更新 2025年7月9日 - 現状 2025年7月9日 処理サイト : PDBe / 状態 : 公開
すべて表示 表示を減らす
 データを開く
データを開く 基本情報
基本情報
 マップデータ
マップデータ 試料
試料 キーワード
キーワード 機能・相同性情報
機能・相同性情報 Homo sapiens (ヒト)
Homo sapiens (ヒト) データ登録者
データ登録者 英国, 1件
英国, 1件  引用
引用 ジャーナル: Nat Commun / 年: 2022
ジャーナル: Nat Commun / 年: 2022
 構造の表示
構造の表示 ダウンロードとリンク
ダウンロードとリンク emd_14875.map.gz
emd_14875.map.gz EMDBマップデータ形式
EMDBマップデータ形式 emd-14875-v30.xml
emd-14875-v30.xml emd-14875.xml
emd-14875.xml EMDBヘッダ
EMDBヘッダ emd_14875_fsc.xml
emd_14875_fsc.xml FSCデータファイル
FSCデータファイル emd_14875.png
emd_14875.png emd-14875.cif.gz
emd-14875.cif.gz emd_14875_half_map_1.map.gz
emd_14875_half_map_1.map.gz emd_14875_half_map_2.map.gz
emd_14875_half_map_2.map.gz http://ftp.pdbj.org/pub/emdb/structures/EMD-14875
http://ftp.pdbj.org/pub/emdb/structures/EMD-14875 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-14875
ftp://ftp.pdbj.org/pub/emdb/structures/EMD-14875 emd_14875_validation.pdf.gz
emd_14875_validation.pdf.gz EMDB検証レポート
EMDB検証レポート emd_14875_full_validation.pdf.gz
emd_14875_full_validation.pdf.gz emd_14875_validation.xml.gz
emd_14875_validation.xml.gz emd_14875_validation.cif.gz
emd_14875_validation.cif.gz https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-14875
https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-14875 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-14875
ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-14875 リンク
リンク EMDB (EBI/PDBe) /
EMDB (EBI/PDBe) /  EMDataResource
EMDataResource マップ
マップ ダウンロード / ファイル: emd_14875.map.gz / 形式: CCP4 / 大きさ: 134.6 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES)
ダウンロード / ファイル: emd_14875.map.gz / 形式: CCP4 / 大きさ: 134.6 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) 試料の構成要素
試料の構成要素 Homo sapiens (ヒト)
Homo sapiens (ヒト) Homo sapiens (ヒト)
Homo sapiens (ヒト) Spodoptera (蝶・蛾)
Spodoptera (蝶・蛾) Homo sapiens (ヒト)
Homo sapiens (ヒト) Spodoptera (蝶・蛾)
Spodoptera (蝶・蛾) Homo sapiens (ヒト)
Homo sapiens (ヒト) Spodoptera (蝶・蛾)
Spodoptera (蝶・蛾)
 解析
解析 試料調製
試料調製 電子顕微鏡法
電子顕微鏡法 FIELD EMISSION GUN
FIELD EMISSION GUN
 ムービー
ムービー コントローラー
コントローラー

































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)