[English] 日本語
 Yorodumi
Yorodumi- EMDB-14856: Mammalian Dicer in the "pre-dicing state" with pre-miR-15a substr... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Mammalian Dicer in the "pre-dicing state" with pre-miR-15a substrate and TARBP2 subunit | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | endoribonuclease / dsRNA / complex / gene silencing / post-transcriptional / catalytic complex / cytoplasm / RISC-loading complex / TARBP2 RNA BINDING PROTEIN / RNA BINDING PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of muscle cell apoptotic process / MicroRNA (miRNA) biogenesis / Small interfering RNA (siRNA) biogenesis / ganglion development / regulation of siRNA processing / regulation of miRNA processing / hair follicle cell proliferation / zygote asymmetric cell division / cardiac neural crest cell development involved in outflow tract morphogenesis / regulation of oligodendrocyte differentiation ...regulation of muscle cell apoptotic process / MicroRNA (miRNA) biogenesis / Small interfering RNA (siRNA) biogenesis / ganglion development / regulation of siRNA processing / regulation of miRNA processing / hair follicle cell proliferation / zygote asymmetric cell division / cardiac neural crest cell development involved in outflow tract morphogenesis / regulation of oligodendrocyte differentiation / olfactory bulb interneuron differentiation / negative regulation of cytoplasmic pattern recognition receptor signaling pathway / regulation of odontogenesis of dentin-containing tooth / regulation of viral transcription / regulation of enamel mineralization / regulation of miRNA metabolic process / peripheral nervous system myelin formation / negative regulation of defense response to virus by host / trophectodermal cell proliferation / spermatogonial cell division / regulation of RNA metabolic process / regulation of epithelial cell differentiation / PKR-mediated signaling / global gene silencing by mRNA cleavage / pre-miRNA binding / spinal cord motor neuron differentiation / negative regulation of Schwann cell proliferation / epidermis morphogenesis / reproductive structure development / ribonuclease III / myoblast differentiation involved in skeletal muscle regeneration / regulation of Notch signaling pathway / regulation of regulatory T cell differentiation / positive regulation of Schwann cell differentiation / nerve development / RISC-loading complex / positive regulation of myelination / meiotic spindle organization / intestinal epithelial cell development / RISC complex assembly / miRNA processing / regulatory ncRNA-mediated post-transcriptional gene silencing / ribonuclease III activity / skeletal muscle tissue regeneration / pre-miRNA processing / siRNA processing / pericentric heterochromatin formation / siRNA binding / regulation of stem cell differentiation / regulation of viral genome replication / RISC complex / mRNA stabilization / inner ear receptor cell development / cartilage development / embryonic limb morphogenesis / embryonic hindlimb morphogenesis / digestive tract development / positive regulation of miRNA metabolic process / cardiac muscle cell development / regulation of neuron differentiation / miRNA binding / neural precursor cell proliferation / regulation of myelination / negative regulation of glial cell proliferation / hair follicle morphogenesis / positive regulation of muscle cell differentiation / stem cell population maintenance / branching morphogenesis of an epithelial tube / spermatid development / hair follicle development / regulation of neurogenesis / single fertilization / RNA processing / postsynaptic density, intracellular component / positive regulation of viral genome replication / spleen development / neuron projection morphogenesis / spindle assembly / protein sequestering activity / lung development / positive regulation of translation / post-embryonic development / helicase activity / cerebral cortex development / multicellular organism growth / rRNA processing / double-stranded RNA binding / regulation of gene expression / regulation of inflammatory response / angiogenesis / gene expression / defense response to virus / cell population proliferation / regulation of cell cycle / nuclear body / positive regulation of gene expression / perinuclear region of cytoplasm / glutamatergic synapse / enzyme binding / negative regulation of transcription by RNA polymerase II Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.81 Å | |||||||||
 Authors Authors | Zanova M / Zapletal D / Kubicek K / Stefl R / Pinkas M / Novacek J | |||||||||
| Funding support |  Czech Republic, 2 items Czech Republic, 2 items
| |||||||||
 Citation Citation |  Journal: Mol Cell / Year: 2022 Journal: Mol Cell / Year: 2022Title: Structural and functional basis of mammalian microRNA biogenesis by Dicer. Authors: David Zapletal / Eliska Taborska / Josef Pasulka / Radek Malik / Karel Kubicek / Martina Zanova / Christian Much / Marek Sebesta / Valeria Buccheri / Filip Horvat / Irena Jenickova / ...Authors: David Zapletal / Eliska Taborska / Josef Pasulka / Radek Malik / Karel Kubicek / Martina Zanova / Christian Much / Marek Sebesta / Valeria Buccheri / Filip Horvat / Irena Jenickova / Michaela Prochazkova / Jan Prochazka / Matyas Pinkas / Jiri Novacek / Diego F Joseph / Radislav Sedlacek / Carrie Bernecky / Dónal O'Carroll / Richard Stefl / Petr Svoboda /      Abstract: MicroRNA (miRNA) and RNA interference (RNAi) pathways rely on small RNAs produced by Dicer endonucleases. Mammalian Dicer primarily supports the essential gene-regulating miRNA pathway, but how it is ...MicroRNA (miRNA) and RNA interference (RNAi) pathways rely on small RNAs produced by Dicer endonucleases. Mammalian Dicer primarily supports the essential gene-regulating miRNA pathway, but how it is specifically adapted to miRNA biogenesis is unknown. We show that the adaptation entails a unique structural role of Dicer's DExD/H helicase domain. Although mice tolerate loss of its putative ATPase function, the complete absence of the domain is lethal because it assures high-fidelity miRNA biogenesis. Structures of murine Dicer•-miRNA precursor complexes revealed that the DExD/H domain has a helicase-unrelated structural function. It locks Dicer in a closed state, which facilitates miRNA precursor selection. Transition to a cleavage-competent open state is stimulated by Dicer-binding protein TARBP2. Absence of the DExD/H domain or its mutations unlocks the closed state, reduces substrate selectivity, and activates RNAi. Thus, the DExD/H domain structurally contributes to mammalian miRNA biogenesis and underlies mechanistical partitioning of miRNA and RNAi pathways. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_14856.map.gz emd_14856.map.gz | 117.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-14856-v30.xml emd-14856-v30.xml emd-14856.xml emd-14856.xml | 24.6 KB 24.6 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_14856.png emd_14856.png | 70.1 KB | ||
| Filedesc metadata |  emd-14856.cif.gz emd-14856.cif.gz | 8.1 KB | ||
| Others |  emd_14856_half_map_1.map.gz emd_14856_half_map_1.map.gz emd_14856_half_map_2.map.gz emd_14856_half_map_2.map.gz | 199.8 MB 199.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-14856 http://ftp.pdbj.org/pub/emdb/structures/EMD-14856 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-14856 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-14856 | HTTPS FTP |
-Related structure data
| Related structure data |  7zpkMC  7yymC  7yynC  7yz4C  7zpiC  7zpjC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_14856.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_14856.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.835 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_14856_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
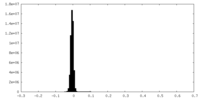
| Density Histograms |
-Half map: #1
| File | emd_14856_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
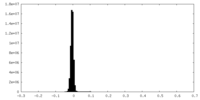
| Density Histograms |
- Sample components
Sample components
-Entire : Pre-dicing state of mouse somatic dicer with pre-mir-15a and TARBP2
| Entire | Name: Pre-dicing state of mouse somatic dicer with pre-mir-15a and TARBP2 |
|---|---|
| Components |
|
-Supramolecule #1: Pre-dicing state of mouse somatic dicer with pre-mir-15a and TARBP2
| Supramolecule | Name: Pre-dicing state of mouse somatic dicer with pre-mir-15a and TARBP2 type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|
-Supramolecule #2: Pre-dicing state of mouse somatic dicer with TARBP2
| Supramolecule | Name: Pre-dicing state of mouse somatic dicer with TARBP2 / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1, #3 |
|---|---|
| Source (natural) | Organism:  |
-Supramolecule #3: Pre-mir-15a
| Supramolecule | Name: Pre-mir-15a / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Endoribonuclease Dicer
| Macromolecule | Name: Endoribonuclease Dicer / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO / EC number: ribonuclease III |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 226.925656 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MVWSHPQFEK GGGSGGGSGG SAWSHPQFEK GDYPYDVPDY AGTENLYFQG LVDMKSPALQ PLSMAGLQLM TPASSPMGPF FGLPWQQEA IHDNIYTPRK YQVELLEAAL DHNTIVCLNT GSGKTFIAVL LTKELAHQIR GDLNPHAKRT VFLVNSANQV A QQVSAVRT ...String: MVWSHPQFEK GGGSGGGSGG SAWSHPQFEK GDYPYDVPDY AGTENLYFQG LVDMKSPALQ PLSMAGLQLM TPASSPMGPF FGLPWQQEA IHDNIYTPRK YQVELLEAAL DHNTIVCLNT GSGKTFIAVL LTKELAHQIR GDLNPHAKRT VFLVNSANQV A QQVSAVRT HSDLKVGEYS DLEVNASWTK ERWSQEFTKH QVLIMTCYVA LTVLKNGYLS LSDINLLVFD ECHLAILDHP YR EIMKLCE SCPSCPRILG LTASILNGKC DPEELEEKIQ KLERILRSDA ETATDLVVLD RYTSQPCEIV VDCGPFTDRS GLY ERLLME LEAALDFIND CNVAVHSKER DSTLISKQIL SDCRAVLVVL GPWCADKVAG MMVRELQKYI KHEQEELHRK FLLF TDTLL RKIHALCEEY FSPASLDLKY VTPKVMKLLE ILRKYKPYER QQFESVEWYN NRNQDNYVSW SDSEDDDDDE EIEEK EKPE TNFPSPFTNI LCGIIFVERR YTAVVLNRLI KEAGKQDPEL AYISSNFITG HGIGKNQPRS KQMEAEFRKQ EEVLRK FRA HETNLLIATS VVEEGVDIPK CNLVVRFDLP TEYRSYVQSK GRARAPISNY VMLADTDKIK SFEEDLKTYK AIEKILR NK CSKSADGAEA DVHAGVDDED AFPPYVLRPD DGGPRVTINT AIGHINRYCA RLPSDPFTHL APKCRTRELP DGTFYSTL Y LPINSPLRAS IVGPPMDSVR LAERVVALIC CEKLHKIGEL DEHLMPVGKE TVKYEEELDL HDEEETSVPG RPGSTKRRQ CYPKAIPECL RESYPKPDQP CYLYVIGMVL TTPLPDELNF RRRKLYPPED TTRCFGILTA KPIPQIPHFP VYTRSGEVTI SIELKKSGF TLSQQMLELI TRLHQYIFSH ILRLEKPALE FKPTGAESAY CVLPLNVVND SGTLDIDFKF MEDIEKSEAR I GIPSTKYS KETPFVFKLE DYQDAVIIPR YRNFDQPHRF YVADVYTDLT PLSKFPSPEY ETFAEYYKTK YNLDLTNLNQ PL LDVDHTS SRLNLLTPRH LNQKGKALPL SSAEKRKAKW ESLQNKQILV PELCAIHPIP ASLWRKAVCL PSILYRLHCL LTA EELRAQ TASDAGVGVR SLPVDFRYPN LDFGWKKSID SKSFISSCNS SLAESDNYCK HSTTVVPEHA AHQGATRPSL ENHD QMSVN CKRLPAESPA KLQSEVSTDL TAINGLSYNK NLANGSYDLV NRDFCQGNQL NYFKQEIPVQ PTTSYPIQNL YNYEN QPKP SNECPLLSNT YLDGNANTST SDGSPAVSTM PAMMNAVKAL KDRMDSEQSP SVGYSSRTLG PNPGLILQAL TLSNAS DGF NLERLEMLGD SFLKHAITTY LFCTYPDAHE GRLSYMRSKK VSNCNLYRLG KKKGLPSRMV VSIFDPPVNW LPPGYVV NQ DKSNSEKWEK DEMTKDCLLA NGKLGEACEE EEDLTWRAPK EEAEDEDDFL EYDQEHIQFI DSMLMGSGAF VRKISLSP F SASDSAYEWK MPKKASLGSM PFASGLEDFD YSSWDAMCYL DPSKAVEEDD FVVGFWNPSE ENCGVDTGKQ SISYDLHTE QCIADKSIAD CVEALLGCYL TSCGERAAQL FLCSLGLKVL PVIKRTSREK ALDPAQENGS SQQKSLSGSC ASPVGPRSSA GKDLEYGCL KIPPRCMFDH PDAEKTLNHL ISGFETFEKK INYRFKNKAY LLQAFTHASY HYNTITDCYQ RLEFLGDAIL D YLITKHLY EDPRQHSPGV LTDLRSALVN NTIFASLAVK YDYHKYFKAV SPELFHVIDD FVKFQLEKNE MQGMDSELRR SE EDEEKEE DIEVPKAMGD IFESLAGAIY MDSGMSLEVV WQVYYPMMQP LIEKFSANVP RSPVRELLEM EPETAKFSPA ERT YDGKVR VTVEVVGKGK FKGVGRSYRI AKSAAARRAL RSLKANQPQV PNSGRGENLY FQGASDYKDH DGDYKDHDGS HHHH HHHH UniProtKB: Endoribonuclease Dicer |
-Macromolecule #3: RISC-loading complex subunit TARBP2
| Macromolecule | Name: RISC-loading complex subunit TARBP2 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 39.991059 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSEEDQGSGT TTGCGLPSIE QMLAANPGKT PISLLQEYGT RIGKTPVYDL LKAEGQAHQP NFTFRVTVGD TSCTGQGPSK KAAKHKAAE VALKHLKGGS MLEPALEDSS SFSLLDSSPP EDTPVVAAEA AAPVPSAVLT RSPPMEMQPP VSPQQSECNP V GALQELVV ...String: MSEEDQGSGT TTGCGLPSIE QMLAANPGKT PISLLQEYGT RIGKTPVYDL LKAEGQAHQP NFTFRVTVGD TSCTGQGPSK KAAKHKAAE VALKHLKGGS MLEPALEDSS SFSLLDSSPP EDTPVVAAEA AAPVPSAVLT RSPPMEMQPP VSPQQSECNP V GALQELVV QKGWRLPEYM VTQESGPAHR KEFTMTCRVE RFIEIGSGTS KKLAKRNAAA KMLLRVHTVP LDARDGNEAE PD DDHFSIG VSSRLDGLRN RGPGCTWDSL RNSVGEKILS LRSCSVGSLG ALGSACCSVL SELSEEQAFH VSYLDIEELS LSG LCQCLV ELSTQPATVC YGSATTREAA RGDAAHRALQ YLRIMAGSKH HHHHHHH UniProtKB: RISC-loading complex subunit TARBP2 |
-Macromolecule #2: 59-nt precursor of miR-15a
| Macromolecule | Name: 59-nt precursor of miR-15a / type: rna / ID: 2 / Number of copies: 1 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 19.017279 KDa |
| Sequence | String: UAGCAGCACA UAAUGGUUUG UGGAUGUUGA AAAGGUGCAG GCCAUACUGU GCUGCCUCA GENBANK: GENBANK: NR_029733.1 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.2 mg/mL | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
Details: The buffer was always prepared fresh in RNAse-free manner. | ||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277.15 K / Instrument: FEI VITROBOT MARK IV / Details: Described in STAR methods. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Detector mode: COUNTING / Number real images: 48253 / Average electron dose: 60.198 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 3.5 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model |
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Details | Initial model used from PDB ID:7ZPJ TARBP2 dsRBD domain 1: initial model from PDB ID:7ZPJ TARBP2 dsRBD domains 2,3: manually fitted into EM map according to map density | ||||||||
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT / Overall B value: 189.2 / Target criteria: Correlation coefficient | ||||||||
| Output model |  PDB-7zpk: |
 Movie
Movie Controller
Controller









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)