[English] 日本語
 Yorodumi
Yorodumi- EMDB-13042: La Crosse virus polymerase at transcription early-elongation stage -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-13042 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | La Crosse virus polymerase at transcription early-elongation stage | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | RNA-dependent RNA polymerase / VIRAL PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationhost cell endoplasmic reticulum / virion component / host cell endoplasmic reticulum-Golgi intermediate compartment / host cell Golgi apparatus / Hydrolases; Acting on ester bonds / hydrolase activity / RNA-directed RNA polymerase / viral RNA genome replication / nucleotide binding / RNA-directed RNA polymerase activity ...host cell endoplasmic reticulum / virion component / host cell endoplasmic reticulum-Golgi intermediate compartment / host cell Golgi apparatus / Hydrolases; Acting on ester bonds / hydrolase activity / RNA-directed RNA polymerase / viral RNA genome replication / nucleotide binding / RNA-directed RNA polymerase activity / DNA-templated transcription / RNA binding / metal ion binding Similarity search - Function | |||||||||
| Biological species |  La Crosse virus / La Crosse virus /  Bunyavirus La Crosse Bunyavirus La Crosse | |||||||||
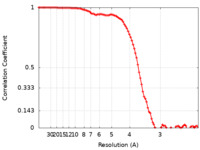
| Method | single particle reconstruction / cryo EM / Resolution: 3.3 Å | |||||||||
 Authors Authors | Arragain B / Durieux Trouilleton Q | |||||||||
| Funding support |  France, 1 items France, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2022 Journal: Nat Commun / Year: 2022Title: Structural snapshots of La Crosse virus polymerase reveal the mechanisms underlying Peribunyaviridae replication and transcription. Authors: Benoît Arragain / Quentin Durieux Trouilleton / Florence Baudin / Jan Provaznik / Nayara Azevedo / Stephen Cusack / Guy Schoehn / Hélène Malet /   Abstract: Segmented negative-strand RNA bunyaviruses encode a multi-functional polymerase that performs genome replication and transcription. Here, we establish conditions for in vitro activity of La Crosse ...Segmented negative-strand RNA bunyaviruses encode a multi-functional polymerase that performs genome replication and transcription. Here, we establish conditions for in vitro activity of La Crosse virus polymerase and visualize its conformational dynamics by cryo-electron microscopy, unveiling the precise molecular mechanics underlying its essential activities. We find that replication initiation is coupled to distal duplex promoter formation, endonuclease movement, prime-and-realign loop extension and closure of the polymerase core that direct the template towards the active site. Transcription initiation depends on C-terminal region closure and endonuclease movements that prompt primer cleavage prior to primer entry in the active site. Product realignment after priming, observed in replication and transcription, is triggered by the prime-and-realign loop. Switch to elongation results in polymerase reorganization and core region opening to facilitate template-product duplex formation in the active site cavity. The uncovered detailed mechanics should be helpful for the future design of antivirals counteracting bunyaviral life threatening pathogens. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_13042.map.gz emd_13042.map.gz | 6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-13042-v30.xml emd-13042-v30.xml emd-13042.xml emd-13042.xml | 21.3 KB 21.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_13042_fsc.xml emd_13042_fsc.xml | 9.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_13042.png emd_13042.png | 137.4 KB | ||
| Filedesc metadata |  emd-13042.cif.gz emd-13042.cif.gz | 8.1 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-13042 http://ftp.pdbj.org/pub/emdb/structures/EMD-13042 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13042 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13042 | HTTPS FTP |
-Related structure data
| Related structure data |  7ormMC  7oriC  7orjC  7orkC  7orlC  7ornC  7oroC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-11022 (Title: Cryo-EM data used for the determination of LACV-L structure in transcription early-elongation state EMPIAR-11022 (Title: Cryo-EM data used for the determination of LACV-L structure in transcription early-elongation stateData size: 721.4 Data #1: Unaligned multiframe micrographs of LACV-L incubated with capped RNA 14-mer, 3' vRNA 1-25, 5' 1-17BPm, UTP, ATP, GTP, MgCl2 [micrographs - multiframe]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_13042.map.gz / Format: CCP4 / Size: 67 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_13042.map.gz / Format: CCP4 / Size: 67 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.145 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : La Crosse virus polymerase at transcription early elongation
| Entire | Name: La Crosse virus polymerase at transcription early elongation |
|---|---|
| Components |
|
-Supramolecule #1: La Crosse virus polymerase at transcription early elongation
| Supramolecule | Name: La Crosse virus polymerase at transcription early elongation type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#4 Details: La Crosse virus polymerase at transcription early elongation stage in complex with the 5prime promoter, the 3prime promoter and template and the capped product RNA formed |
|---|---|
| Source (natural) | Organism:  La Crosse virus La Crosse virus |
| Molecular weight | Theoretical: 287 KDa |
-Macromolecule #1: RNA (5'-R(P*AP*CP*GP*AP*GP*UP*GP*UP*CP*GP*UP*AP*CP*C)-3')
| Macromolecule | Name: RNA (5'-R(P*AP*CP*GP*AP*GP*UP*GP*UP*CP*GP*UP*AP*CP*C)-3') type: rna / ID: 1 Details: 5prime vRNA of La Crosse M segment. Nucleotides G2, U3, A9 and C10 were mutated into C2, G3, C9 and G10 Number of copies: 1 |
|---|---|
| Source (natural) | Organism:  La Crosse virus La Crosse virus |
| Molecular weight | Theoretical: 5.466325 KDa |
| Sequence | String: ACGAGUGUCG UACCAAG |
-Macromolecule #2: RNA (5'-R(P*AP*AP*AP*GP*UP*AP*CP*AP*CP*UP*AP*CP*U)-3')
| Macromolecule | Name: RNA (5'-R(P*AP*AP*AP*GP*UP*AP*CP*AP*CP*UP*AP*CP*U)-3') type: rna / ID: 2 / Number of copies: 1 |
|---|---|
| Source (natural) | Organism:  La Crosse virus La Crosse virus |
| Molecular weight | Theoretical: 7.882668 KDa |
| Sequence | String: UAUCUAUACU UGGUAGUACA CUACU |
-Macromolecule #3: RNA (5'-R(P*UP*AP*UP*AP*AP*UP*AP*GP*UP*AP*GP*UP*GP*UP*A)-3')
| Macromolecule | Name: RNA (5'-R(P*UP*AP*UP*AP*AP*UP*AP*GP*UP*AP*GP*UP*GP*UP*A)-3') type: rna / ID: 3 / Number of copies: 1 |
|---|---|
| Source (natural) | Organism:  La Crosse virus La Crosse virus |
| Molecular weight | Theoretical: 6.417856 KDa |
| Sequence | String: AAUGCUAUAA UAGUAGUGUA |
-Macromolecule #4: RNA-directed RNA polymerase L
| Macromolecule | Name: RNA-directed RNA polymerase L / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO / EC number: RNA-directed RNA polymerase |
|---|---|
| Source (natural) | Organism:  Bunyavirus La Crosse Bunyavirus La Crosse |
| Molecular weight | Theoretical: 264.751062 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MDYQEYQQFL ARINTARDAC VAKDIDVDLL MARKDYFGRE LCKSLNIEYR NDVPFIDIIL DIRPEVDPLT IDAPHITPDN YLYINNVLY IIDYKVSVSN ESSVITYDKY YELTRDISDR LSIPIEIVII RIDPVSRDLH INSDRFKELY PTIVVDINFN Q FFDLKQLL ...String: MDYQEYQQFL ARINTARDAC VAKDIDVDLL MARKDYFGRE LCKSLNIEYR NDVPFIDIIL DIRPEVDPLT IDAPHITPDN YLYINNVLY IIDYKVSVSN ESSVITYDKY YELTRDISDR LSIPIEIVII RIDPVSRDLH INSDRFKELY PTIVVDINFN Q FFDLKQLL YEKFGDDEEF LLKVAHGDFT LTAPWCKTGC PEFWKHPIYK EFKMSMPVPE RRLFEESVKF NAYESERWNT NL VKIREYT KKDYSEHISK SAKNIFLASG FYKQPNKNEI SEGWTLMVER VQDQREISKS LHDQKPSIHF IWGAHNPGNS NNA TFKLIL LSKSLQSIKG ISTYTEAFKS LGKMMDIGDK AIEYEEFCMS LKSKARSSWK QIMNKKLEPK QINNALVLWE QQFM INNDL IDKSEKLKLF KNFCGIGKHK QFKNKMLEDL EVSKPKILDF DDANMYLASL TMMEQSKKIL SKSNGLKPDN FILNE FGSR IKDANKETYD NMHKIFETGY WQCISDFSTL MKNILSVSQY NRHNTFRIAM CANNNVFAIV FPSADIKTKK ATVVYS IIV LHKEEENIFN PGCLHGTFKC MNGYISISRA IRLDKERCQR IVSSPGLFLT TCLLFKHDNP TLVMSDIMNF SIYTSLS IT KSVLSLTEPA RYMIMNSLAI SSNVKDYIAE KFSPYTKTLF SVYMTRLIKN ACFDAYDQRQ RVQLRDIYLS DYDITQKG I KDNRELTSIW FPGSVTLKEY LTQIYLPFYF NAKGLHEKHH VMVDLAKTIL EIECEQRENI KEIWSTNCTK QTVNLKILI HSLCKNLLAD TSRHNHLRNR IENRNNFRRS ITTISTFTSS KSCLKIGDFR KEKELQSVKQ KKILEVQSRK MRLANPMFVT DEQVCLEVG HCNYEMLRNA MPNYTDYIST KVFDRLYELL DKKVLTDKPV IEQIMDMMID HKKFYFTFFN KGQKTSKDRE I FVGEYEAK MCMYAVERIA KERCKLNPDE MISEPGDGKL KVLEQKSEQE IRFLVETTRQ KNREIDEAIE ALATEGSGWS HP QFEKGSG YESNLGKIEK LSLGKAKGLK MEINADMSKW SAQDVFYKYF WLIALDPILY PQEKERILYF MCNYMDKELI LPD ELLFNL LDQKVAYQND IIATMTNQLN SNTVLIKRNW LQGNFNYTSS YVHSCAMSVY KEILKEAITL LDGSILVNSL VHSD DNQTS ITIVQDKMEN DKIIDFAMKE FERACLTFGC QANMKKTYVT NCIKEFVSLF NLYGEPFSIY GRFLLTSVGD CAYIG PYED LASRISSAQT AIKHGCPPSL AWVSIAISHW MTSLTYNMLP GQSNDPIDYF PAENRKDIPI ELNGVLDAPL SMISTV GLE SGNLYFLIKL LSKYTPVMQK RESVVNQIAE VKNWKVEDLT DNEIFRLKIL RYLVLDAEMD PSDIMGETSD MRGRSIL TP RKFTTAGSLR KLYSFSKYQD RLSSPGGMVE LFTYLLEKPE LLVTKGEDMK DYMESVIFRY NSKRFKESLS IQNPAQLF I EQILFSHKPV IDFSGIRDKY INLHDSRALE KEPDILGKVT FTEAYRLLMR DLSSLELTND DIQVIYSYII LNDPMMITI ANTHILSIYG SPQRRMGMSC STMPEFRNLK LIHHSPALVL RAYSKNNPDI QGADPTEMAR DLVHLKEFVE NTNLEEKMKV RIAMNEAEK GQRDIVFELK EMTRFYQVCY EYVKSTEHKI KVFILPAKSY TTTDFCSLMQ GNLIKDKEWY TVHYLKQILS G GHKAIMQH NATSEQNIAF ECFKLITHFA DSFIDSLSRS AFLQLIIDEF SYKDVKVSKL YDIIKNGYNR TDFIPLLFRT GD LRQADLD KYDAMKSHER VTWNDWQTSR HLDMGSINLT ITGYNRSITI IGEDNKLTYA ELCLTRKTPE NITISGRKLL GSR HGLKFE NMSKIQTYPG NYYITYRKKD RHQFVYQIHS HESITRRNEE HMAIRTRIYN EITPVCVVNV AEVDGDQRIL IRSL DYLNN DIFSLSRIKV GLDEFATIKK AHFSKMVSFE GPPIKTGLLD LTELMKSQDL LNLNYDNIRN SNLISFSKLI CCEGS DNIN DGLEFLSDDP MNFTEGEAIH STPIFNIYYS KRGERHMTYR NAIKLLIERE TKIFEEAFTF SENGFISPEN LGCLEA VVS LIKLLKTNEW STVIDKCIHI CLIKNGMDHM YHSFDVPKCF MGNPITRDIN WVMFREFINS LPGTDIPPWN VMTENFK KK CIALINSKFE TQRDFSEFTK LMKKEGGRSN IEFD UniProtKB: RNA-directed RNA polymerase L |
-Macromolecule #5: ZINC ION
| Macromolecule | Name: ZINC ION / type: ligand / ID: 5 / Number of copies: 1 / Formula: ZN |
|---|---|
| Molecular weight | Theoretical: 65.409 Da |
-Macromolecule #6: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 6 / Number of copies: 1 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.45 mg/mL | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| ||||||||||||||||
| Grid | Model: UltrAuFoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Support film - Material: GOLD / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 45 sec. / Details: 25mA | ||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 293 K / Instrument: FEI VITROBOT MARK IV | ||||||||||||||||
| Details | 1.3 uM LACV-LCItag_H34K were sequentially incubated for 1h at 4 degree with (i) 1.9 uM 5prime 1-17 BPm and 3.9 uM commercial 14-mer capped primer, (ii) 1.9 uM 3prime vRNA 1-25. LACV-LCItag_H34K bound to vRNAs and capped primer was incubated with 100 uM ATP/GTP/UTP and 2mM MgCl2 for 1h at 30 degree. |
- Electron microscopy
Electron microscopy
| Microscope | TFS GLACIOS |
|---|---|
| Temperature | Min: 70.0 K / Max: 70.0 K |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Digitization - Frames/image: 3-50 / Number grids imaged: 1 / Number real images: 2524 / Average exposure time: 6.6 sec. / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Calibrated defocus max: 2.0 µm / Calibrated defocus min: 0.8 µm / Calibrated magnification: 36000 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 36000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Chain ID: A / Chain - Residue range: 1-2263 / Chain - Source name: PDB / Chain - Initial model type: experimental model |
|---|---|
| Refinement | Space: REAL / Protocol: AB INITIO MODEL / Overall B value: 61.26 |
| Output model |  PDB-7orm: |
 Movie
Movie Controller
Controller

















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)