[English] 日本語
 Yorodumi
Yorodumi- EMDB-0869: Cryo-EM structure of the MgtE Mg2+ channel under Mg2+-free conditions -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-0869 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the MgtE Mg2+ channel under Mg2+-free conditions | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationmagnesium ion transport / magnesium ion transmembrane transporter activity / magnesium ion binding / protein homodimerization activity / identical protein binding / plasma membrane Similarity search - Function | |||||||||
| Biological species |   Thermus thermophilus (bacteria) / Thermus thermophilus (bacteria) /   Thermus thermophilus HB8 (bacteria) / Thermus thermophilus HB8 (bacteria) /  | |||||||||
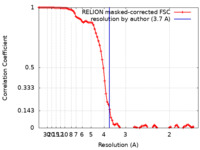
| Method | single particle reconstruction / cryo EM / Resolution: 3.7 Å | |||||||||
 Authors Authors | Hattori M / Jin F | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: PLoS Biol / Year: 2021 Journal: PLoS Biol / Year: 2021Title: The structure of MgtE in the absence of magnesium provides new insights into channel gating. Authors: Fei Jin / Minxuan Sun / Takashi Fujii / Yurika Yamada / Jin Wang / Andrés D Maturana / Miki Wada / Shichen Su / Jinbiao Ma / Hironori Takeda / Tsukasa Kusakizako / Atsuhiro Tomita / Yoshiko ...Authors: Fei Jin / Minxuan Sun / Takashi Fujii / Yurika Yamada / Jin Wang / Andrés D Maturana / Miki Wada / Shichen Su / Jinbiao Ma / Hironori Takeda / Tsukasa Kusakizako / Atsuhiro Tomita / Yoshiko Nakada-Nakura / Kehong Liu / Tomoko Uemura / Yayoi Nomura / Norimichi Nomura / Koichi Ito / Osamu Nureki / Keiichi Namba / So Iwata / Ye Yu / Motoyuki Hattori /   Abstract: MgtE is a Mg2+ channel conserved in organisms ranging from prokaryotes to eukaryotes, including humans, and plays an important role in Mg2+ homeostasis. The previously determined MgtE structures in ...MgtE is a Mg2+ channel conserved in organisms ranging from prokaryotes to eukaryotes, including humans, and plays an important role in Mg2+ homeostasis. The previously determined MgtE structures in the Mg2+-bound, closed-state, and structure-based functional analyses of MgtE revealed that the binding of Mg2+ ions to the MgtE cytoplasmic domain induces channel inactivation to maintain Mg2+ homeostasis. There are no structures of the transmembrane (TM) domain for MgtE in Mg2+-free conditions, and the pore-opening mechanism has thus remained unclear. Here, we determined the cryo-electron microscopy (cryo-EM) structure of the MgtE-Fab complex in the absence of Mg2+ ions. The Mg2+-free MgtE TM domain structure and its comparison with the Mg2+-bound, closed-state structure, together with functional analyses, showed the Mg2+-dependent pore opening of MgtE on the cytoplasmic side and revealed the kink motions of the TM2 and TM5 helices at the glycine residues, which are important for channel activity. Overall, our work provides structure-based mechanistic insights into the channel gating of MgtE. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_0869.map.gz emd_0869.map.gz | 6.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-0869-v30.xml emd-0869-v30.xml emd-0869.xml emd-0869.xml | 12 KB 12 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_0869_fsc.xml emd_0869_fsc.xml | 9.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_0869.png emd_0869.png | 257.2 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-0869 http://ftp.pdbj.org/pub/emdb/structures/EMD-0869 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0869 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0869 | HTTPS FTP |
-Validation report
| Summary document |  emd_0869_validation.pdf.gz emd_0869_validation.pdf.gz | 366.7 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_0869_full_validation.pdf.gz emd_0869_full_validation.pdf.gz | 366.2 KB | Display | |
| Data in XML |  emd_0869_validation.xml.gz emd_0869_validation.xml.gz | 10.8 KB | Display | |
| Data in CIF |  emd_0869_validation.cif.gz emd_0869_validation.cif.gz | 14 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0869 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0869 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0869 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0869 | HTTPS FTP |
-Related structure data
| Related structure data |  6lbhMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_0869.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_0869.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 0.82 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : MgtE-Fab complex
| Entire | Name: MgtE-Fab complex |
|---|---|
| Components |
|
-Supramolecule #1: MgtE-Fab complex
| Supramolecule | Name: MgtE-Fab complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:   Thermus thermophilus (bacteria) Thermus thermophilus (bacteria) |
| Recombinant expression | Organism:  |
-Macromolecule #1: Magnesium transporter MgtE
| Macromolecule | Name: Magnesium transporter MgtE / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Thermus thermophilus HB8 (bacteria) Thermus thermophilus HB8 (bacteria) |
| Molecular weight | Theoretical: 19.249996 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: LVYSEAGPVA LWLARVRWLV ILILTGMVTS SILQGFESVL EAVTALAFYV PVLLGTGGNT GNQSATLIIR ALATRDLDLR DWRRVFLKE MGVGLLLGLT LSFLLVGKVY WDGHPLLLPV VGVSLVLIVF FANLVGAMLP FLLRRLGVDP ALVSNPLVAT L SDVTGLLI YLSVARLLLE |
-Macromolecule #2: Fab light chain
| Macromolecule | Name: Fab light chain / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 23.837455 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: DVLMTQTPLS LPVSLGDQAS ISCRSSQSLV HSDGNTYLHW YLQKPGQSPK LLIYKVSNRF SGVPDRFSGS GSGTDFTLKI SRVEAEDLG VYFCSQSTHV PWTFGGGTKL EIKRADAAPT VSIFPPSSEQ LTSGGASVVC FLNNFYPKDI NVKWKIDGSE R QNGVLNSW ...String: DVLMTQTPLS LPVSLGDQAS ISCRSSQSLV HSDGNTYLHW YLQKPGQSPK LLIYKVSNRF SGVPDRFSGS GSGTDFTLKI SRVEAEDLG VYFCSQSTHV PWTFGGGTKL EIKRADAAPT VSIFPPSSEQ LTSGGASVVC FLNNFYPKDI NVKWKIDGSE R QNGVLNSW TDQDSKDSTY SMSSTLTLTK DEYERHNSYT CEATHKTSTS PIVKSFNR |
-Macromolecule #3: Fab heavy chain
| Macromolecule | Name: Fab heavy chain / type: protein_or_peptide / ID: 3 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 24.092789 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: EVKLQESGVE LVKPGASVKI SCKASGYSFT GYNMNWVKQS HGKSLEWIGN ISPYYGTSIY NQNFKGKATL TVDRSSSTAY MQLNSLTSE DSAVYYCARG ESFSNYEGYY AMDYWGQGTS VIVSSAKTTA PSVYPLAPVC GDTSGSSVTL GCLVKGYFPE P VTLTWNSG ...String: EVKLQESGVE LVKPGASVKI SCKASGYSFT GYNMNWVKQS HGKSLEWIGN ISPYYGTSIY NQNFKGKATL TVDRSSSTAY MQLNSLTSE DSAVYYCARG ESFSNYEGYY AMDYWGQGTS VIVSSAKTTA PSVYPLAPVC GDTSGSSVTL GCLVKGYFPE P VTLTWNSG SLSSGVHTFP AVLQSDLYTL SSSVTVTSST WPSQSITCNV AHPASSTKVD KKIEPR |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 70.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller