[English] 日本語
 Yorodumi
Yorodumi- EMDB-42386: Cryo-EM map of the human CTF18-RFC-PCNA-DNA ternary complex in th... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
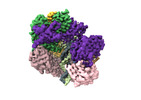
| Title | Cryo-EM map of the human CTF18-RFC-PCNA-DNA ternary complex in the five-subunit binding state (state 4) | |||||||||
 Map data Map data | EM sharpen map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | DNA clamp loader complex / REPLICATION | |||||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of deoxyribonuclease activity / dinucleotide insertion or deletion binding / response to organophosphorus / PCNA-p21 complex / mitotic telomere maintenance via semi-conservative replication / Elg1 RFC-like complex / Ctf18 RFC-like complex / DNA replication factor C complex / purine-specific mismatch base pair DNA N-glycosylase activity / MutLalpha complex binding ...positive regulation of deoxyribonuclease activity / dinucleotide insertion or deletion binding / response to organophosphorus / PCNA-p21 complex / mitotic telomere maintenance via semi-conservative replication / Elg1 RFC-like complex / Ctf18 RFC-like complex / DNA replication factor C complex / purine-specific mismatch base pair DNA N-glycosylase activity / MutLalpha complex binding / positive regulation of DNA-directed DNA polymerase activity / nuclear lamina / DNA clamp loader activity / Polymerase switching / Telomere C-strand (Lagging Strand) Synthesis / Processive synthesis on the lagging strand / PCNA complex / Removal of the Flap Intermediate / Processive synthesis on the C-strand of the telomere / Mismatch repair (MMR) directed by MSH2:MSH3 (MutSbeta) / Polymerase switching on the C-strand of the telomere / Mismatch repair (MMR) directed by MSH2:MSH6 (MutSalpha) / Removal of the Flap Intermediate from the C-strand / Transcription of E2F targets under negative control by DREAM complex / replisome / DNA strand elongation involved in DNA replication / DNA duplex unwinding / response to L-glutamate / HDR through Single Strand Annealing (SSA) / Impaired BRCA2 binding to RAD51 / histone acetyltransferase binding / DNA synthesis involved in DNA repair / leading strand elongation / DNA polymerase processivity factor activity / G1/S-Specific Transcription / response to dexamethasone / replication fork processing / nuclear replication fork / Presynaptic phase of homologous DNA pairing and strand exchange / SUMOylation of DNA replication proteins / PCNA-Dependent Long Patch Base Excision Repair / estrous cycle / mismatch repair / translesion synthesis / ATP-dependent activity, acting on DNA / Activation of ATR in response to replication stress / response to cadmium ion / DNA polymerase binding / cyclin-dependent protein kinase holoenzyme complex / epithelial cell differentiation / base-excision repair, gap-filling / positive regulation of DNA repair / Translesion synthesis by REV1 / Translesion synthesis by POLK / TP53 Regulates Transcription of Genes Involved in G2 Cell Cycle Arrest / Translesion synthesis by POLI / Gap-filling DNA repair synthesis and ligation in GG-NER / positive regulation of DNA replication / male germ cell nucleus / replication fork / nuclear estrogen receptor binding / liver regeneration / Recognition of DNA damage by PCNA-containing replication complex / Termination of translesion DNA synthesis / Translesion Synthesis by POLH / G2/M DNA damage checkpoint / HDR through Homologous Recombination (HRR) / Dual Incision in GG-NER / DNA-templated DNA replication / receptor tyrosine kinase binding / cellular response to hydrogen peroxide / Dual incision in TC-NER / Gap-filling DNA repair synthesis and ligation in TC-NER / cellular response to UV / cellular response to xenobiotic stimulus / E3 ubiquitin ligases ubiquitinate target proteins / response to estradiol / heart development / Processing of DNA double-strand break ends / Regulation of TP53 Activity through Phosphorylation / DNA replication / damaged DNA binding / chromosome, telomeric region / nuclear body / DNA repair / centrosome / chromatin binding / protein-containing complex binding / chromatin / negative regulation of transcription by RNA polymerase II / enzyme binding / ATP hydrolysis activity / DNA binding / extracellular exosome / nucleoplasm / ATP binding / identical protein binding / membrane / nucleus / cytosol Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / synthetic construct (others) Homo sapiens (human) / synthetic construct (others) | |||||||||
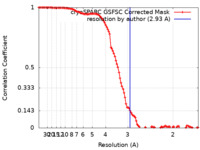
| Method | single particle reconstruction / cryo EM / Resolution: 2.93 Å | |||||||||
 Authors Authors | Wang F / He Q / Li H | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2024 Journal: Proc Natl Acad Sci U S A / Year: 2024Title: Cryo-EM reveals a nearly complete PCNA loading process and unique features of the human alternative clamp loader CTF18-RFC. Authors: Qing He / Feng Wang / Michael E O'Donnell / Huilin Li /  Abstract: The DNA sliding clamp PCNA is a multipurpose platform for DNA polymerases and many other proteins involved in DNA metabolism. The topologically closed PCNA ring needs to be cracked open and loaded ...The DNA sliding clamp PCNA is a multipurpose platform for DNA polymerases and many other proteins involved in DNA metabolism. The topologically closed PCNA ring needs to be cracked open and loaded onto DNA by a clamp loader, e.g., the well-studied pentameric ATPase complex RFC (RFC1-5). The CTF18-RFC complex is an alternative clamp loader found recently to bind the leading strand DNA polymerase ε and load PCNA onto leading strand DNA, but its structure and the loading mechanism have been unknown. By cryo-EM analysis of in vitro assembled human CTF18-RFC-DNA-PCNA complex, we have captured seven loading intermediates, revealing a detailed PCNA loading mechanism onto a 3'-ss/dsDNA junction by CTF18-RFC. Interestingly, the alternative loader has evolved a highly mobile CTF18 AAA+ module likely to lower the loading activity, perhaps to avoid competition with the RFC and to limit its role to leading strand clamp loading. To compensate for the lost stability due to the mobile AAA+ module, CTF18 has evolved a unique β-hairpin motif that reaches across RFC2 to interact with RFC5, thereby stabilizing the pentameric complex. Further, we found that CTF18 also contains a separation pin to locally melt DNA from the 3'-end of the primer; this ensures its ability to load PCNA to any 3'-ss/dsDNA junction, facilitated by the binding energy of the E-plug to the major groove. Our study reveals unique structural features of the human CTF18-RFC and contributes to a broader understanding of PCNA loading by the alternative clamp loaders. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_42386.map.gz emd_42386.map.gz | 118 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-42386-v30.xml emd-42386-v30.xml emd-42386.xml emd-42386.xml | 28.7 KB 28.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_42386_fsc.xml emd_42386_fsc.xml | 10.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_42386.png emd_42386.png | 75.8 KB | ||
| Filedesc metadata |  emd-42386.cif.gz emd-42386.cif.gz | 8.1 KB | ||
| Others |  emd_42386_additional_1.map.gz emd_42386_additional_1.map.gz emd_42386_additional_2.map.gz emd_42386_additional_2.map.gz emd_42386_half_map_1.map.gz emd_42386_half_map_1.map.gz emd_42386_half_map_2.map.gz emd_42386_half_map_2.map.gz | 62.6 MB 110.9 MB 115.9 MB 115.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-42386 http://ftp.pdbj.org/pub/emdb/structures/EMD-42386 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42386 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42386 | HTTPS FTP |
-Validation report
| Summary document |  emd_42386_validation.pdf.gz emd_42386_validation.pdf.gz | 1015 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_42386_full_validation.pdf.gz emd_42386_full_validation.pdf.gz | 1014.5 KB | Display | |
| Data in XML |  emd_42386_validation.xml.gz emd_42386_validation.xml.gz | 19 KB | Display | |
| Data in CIF |  emd_42386_validation.cif.gz emd_42386_validation.cif.gz | 24.5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42386 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42386 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42386 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42386 | HTTPS FTP |
-Related structure data
| Related structure data |  8umwMC  8umtC  8umuC  8umvC  8umyC  8un0C  8unjC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_42386.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_42386.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | EM sharpen map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.828 Å | ||||||||||||||||||||||||||||||||||||
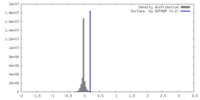
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: Original unsharpen EM map
| File | emd_42386_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Original unsharpen EM map | ||||||||||||
| Projections & Slices |
| ||||||||||||
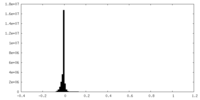
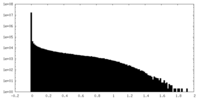
| Density Histograms |
-Additional map: EM sharpen map by DeepEM hancer
| File | emd_42386_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | EM sharpen map by DeepEM hancer | ||||||||||||
| Projections & Slices |
| ||||||||||||
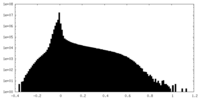
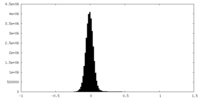
| Density Histograms |
-Half map: EM half map B
| File | emd_42386_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | EM half map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
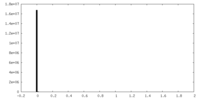
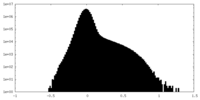
| Density Histograms |
-Half map: EM half map A
| File | emd_42386_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | EM half map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
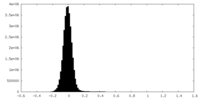
| Density Histograms |
- Sample components
Sample components
+Entire : the human clamp-clamp loader complex PCNA-CTF18
+Supramolecule #1: the human clamp-clamp loader complex PCNA-CTF18
+Macromolecule #1: Chromosome transmission fidelity protein 18 homolog
+Macromolecule #2: Replication factor C subunit 2
+Macromolecule #3: Replication factor C subunit 5
+Macromolecule #4: Replication factor C subunit 4
+Macromolecule #5: Replication factor C subunit 3
+Macromolecule #6: Proliferating cell nuclear antigen
+Macromolecule #7: DNA (40-MER)
+Macromolecule #8: DNA (20-MER)
+Macromolecule #9: MAGNESIUM ION
+Macromolecule #10: PHOSPHOTHIOPHOSPHORIC ACID-ADENYLATE ESTER
+Macromolecule #11: ADENOSINE-5'-DIPHOSPHATE
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.3 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 280 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.9000000000000001 µm / Nominal defocus min: 1.2 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: RIGID BODY FIT / Overall B value: 123 |
|---|---|
| Output model |  PDB-8umw: |
 Movie
Movie Controller
Controller



























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)