+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM Structure of Glutamine Synthetase | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | GS / glutamine synthetase / GLUL / PEPTIDE BINDING PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationAstrocytic Glutamate-Glutamine Uptake And Metabolism / Glutamate and glutamine metabolism / intracellular ammonium homeostasis / protein palmitoylation / protein S-acyltransferase / protein-cysteine S-palmitoyltransferase activity / regulation of sprouting angiogenesis / regulation of endothelial cell migration / glutamine synthetase / : ...Astrocytic Glutamate-Glutamine Uptake And Metabolism / Glutamate and glutamine metabolism / intracellular ammonium homeostasis / protein palmitoylation / protein S-acyltransferase / protein-cysteine S-palmitoyltransferase activity / regulation of sprouting angiogenesis / regulation of endothelial cell migration / glutamine synthetase / : / glutamine synthetase activity / positive regulation of erythrocyte differentiation / angiogenesis / mitochondrion / ATP binding / metal ion binding / plasma membrane / cytoplasm / cytosol Similarity search - Function | |||||||||
| Biological species |  | |||||||||
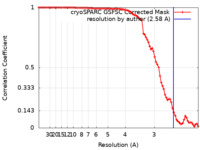
| Method | single particle reconstruction / cryo EM / Resolution: 2.58 Å | |||||||||
 Authors Authors | Morgan CE / Yu EW / Zhang Z | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Cell Rep / Year: 2022 Journal: Cell Rep / Year: 2022Title: Toward structural-omics of the bovine retinal pigment epithelium. Authors: Christopher E Morgan / Zhemin Zhang / Masaru Miyagi / Marcin Golczak / Edward W Yu /  Abstract: The use of an integrated systems biology approach to investigate tissues and organs has been thought to be impracticable in the field of structural biology, where the techniques mainly focus on ...The use of an integrated systems biology approach to investigate tissues and organs has been thought to be impracticable in the field of structural biology, where the techniques mainly focus on determining the structure of a particular biomacromolecule of interest. Here, we report the use of cryoelectron microscopy (cryo-EM) to define the composition of a raw bovine retinal pigment epithelium (RPE) lysate. From this sample, we simultaneously identify and solve cryo-EM structures of seven different RPE enzymes whose functions affect neurotransmitter recycling, iron metabolism, gluconeogenesis, glycolysis, axonal development, and energy homeostasis. Interestingly, dysfunction of these important proteins has been directly linked to several neurodegenerative disorders, including Huntington's disease, amyotrophic lateral sclerosis (ALS), Parkinson's disease, Alzheimer's disease, and schizophrenia. Our work underscores the importance of cryo-EM in facilitating tissue and organ proteomics at the atomic level. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_26356.map.gz emd_26356.map.gz | 7.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-26356-v30.xml emd-26356-v30.xml emd-26356.xml emd-26356.xml | 19.7 KB 19.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_26356_fsc.xml emd_26356_fsc.xml | 8.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_26356.png emd_26356.png | 138.6 KB | ||
| Filedesc metadata |  emd-26356.cif.gz emd-26356.cif.gz | 5.9 KB | ||
| Others |  emd_26356_additional_1.map.gz emd_26356_additional_1.map.gz emd_26356_half_map_1.map.gz emd_26356_half_map_1.map.gz emd_26356_half_map_2.map.gz emd_26356_half_map_2.map.gz | 32.4 MB 59.2 MB 59.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-26356 http://ftp.pdbj.org/pub/emdb/structures/EMD-26356 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26356 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26356 | HTTPS FTP |
-Related structure data
| Related structure data |  7u5nMC  7u5hC  7u5iC  7u5jC  7u5kC  7u5lC  7u5mC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_26356.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_26356.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.07 Å | ||||||||||||||||||||||||||||||||||||
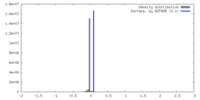
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: #1
| File | emd_26356_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
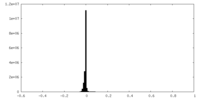
| Density Histograms |
-Half map: #2
| File | emd_26356_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
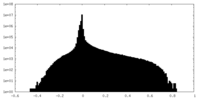
| Density Histograms |
-Half map: #1
| File | emd_26356_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
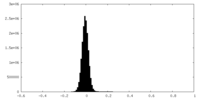
| Density Histograms |
- Sample components
Sample components
-Entire : Decamer of Glutamine Synthetase
| Entire | Name: Decamer of Glutamine Synthetase |
|---|---|
| Components |
|
-Supramolecule #1: Decamer of Glutamine Synthetase
| Supramolecule | Name: Decamer of Glutamine Synthetase / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Glutamine synthetase
| Macromolecule | Name: Glutamine synthetase / type: protein_or_peptide / ID: 1 / Number of copies: 10 / Enantiomer: LEVO / EC number: glutamine synthetase |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 42.085414 KDa |
| Sequence | String: MATSASSHLN KGIKQVYMAL PQGDKVQAMY IWIDGTGEGL RCKTRTLDSE PKCIEELPEW NFDGSSTFQS EGSNSDMYLV PAAMFRDPF RKDPNKLVFC EVFKYNRKPA ETNLRHTCKR IMDMVSNQRP WFGMEQEYTL MGTDGHPFGW PSNGFPGPQG P YYCGVGAD ...String: MATSASSHLN KGIKQVYMAL PQGDKVQAMY IWIDGTGEGL RCKTRTLDSE PKCIEELPEW NFDGSSTFQS EGSNSDMYLV PAAMFRDPF RKDPNKLVFC EVFKYNRKPA ETNLRHTCKR IMDMVSNQRP WFGMEQEYTL MGTDGHPFGW PSNGFPGPQG P YYCGVGAD KAYGRDIVEA HYRACLYAGI KIGGTNAEVM PAQWEFQIGP CEGIDMGDHL WVARFILHRV CEDFGVIATF DP KPIPGNW NGAGCHTNFS TKAMREENGL KYIEEAIEKL SKRHQYHIRA YDPKGGLDNA RRLTGFHETS NINDFSAGVA NRG ASIRIP RTVGQEKKGY FEDRRPSANC DPFAVTEALI RTCLLNETGD EPFQYKN UniProtKB: Glutamine synthetase |
-Macromolecule #2: MANGANESE (II) ION
| Macromolecule | Name: MANGANESE (II) ION / type: ligand / ID: 2 / Number of copies: 10 / Formula: MN |
|---|---|
| Molecular weight | Theoretical: 54.938 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 37.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)