[English] 日本語
 Yorodumi
Yorodumi- EMDB-24953: Cryo-EM structure of TMEM106B fibrils extracted from a FTLD-TDP p... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-24953 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of TMEM106B fibrils extracted from a FTLD-TDP patient, polymorph 1 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | TMEM106B / FTLD-TDP / amyloid / PROTEIN FIBRIL | |||||||||
| Function / homology |  Function and homology information Function and homology informationlysosomal protein catabolic process / lysosomal lumen acidification / regulation of lysosome organization / lysosome localization / positive regulation of dendrite development / dendrite morphogenesis / lysosomal transport / lysosome organization / neuron cellular homeostasis / late endosome membrane ...lysosomal protein catabolic process / lysosomal lumen acidification / regulation of lysosome organization / lysosome localization / positive regulation of dendrite development / dendrite morphogenesis / lysosomal transport / lysosome organization / neuron cellular homeostasis / late endosome membrane / ATPase binding / lysosome / endosome / lysosomal membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
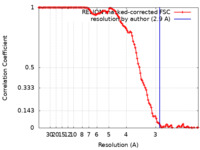
| Method | helical reconstruction / cryo EM / Resolution: 2.9 Å | |||||||||
 Authors Authors | Cao Q / Jiang Y | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2022 Journal: Nature / Year: 2022Title: Amyloid fibrils in FTLD-TDP are composed of TMEM106B and not TDP-43. Authors: Yi Xiao Jiang / Qin Cao / Michael R Sawaya / Romany Abskharon / Peng Ge / Michael DeTure / Dennis W Dickson / Janine Y Fu / Rachel R Ogorzalek Loo / Joseph A Loo / David S Eisenberg /   Abstract: Frontotemporal lobar degeneration (FTLD) is the third most common neurodegenerative condition after Alzheimer's and Parkinson's diseases. FTLD typically presents in 45 to 64 year olds with ...Frontotemporal lobar degeneration (FTLD) is the third most common neurodegenerative condition after Alzheimer's and Parkinson's diseases. FTLD typically presents in 45 to 64 year olds with behavioural changes or progressive decline of language skills. The subtype FTLD-TDP is characterized by certain clinical symptoms and pathological neuronal inclusions with TAR DNA-binding protein (TDP-43) immunoreactivity. Here we extracted amyloid fibrils from brains of four patients representing four of the five FTLD-TDP subclasses, and determined their structures by cryo-electron microscopy. Unexpectedly, all amyloid fibrils examined were composed of a 135-residue carboxy-terminal fragment of transmembrane protein 106B (TMEM106B), a lysosomal membrane protein previously implicated as a genetic risk factor for FTLD-TDP. In addition to TMEM106B fibrils, we detected abundant non-fibrillar aggregated TDP-43 by immunogold labelling. Our observations confirm that FTLD-TDP is associated with amyloid fibrils, and that the fibrils are formed by TMEM106B rather than TDP-43. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_24953.map.gz emd_24953.map.gz | 7.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-24953-v30.xml emd-24953-v30.xml emd-24953.xml emd-24953.xml | 10.9 KB 10.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_24953_fsc.xml emd_24953_fsc.xml | 11.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_24953.png emd_24953.png | 66.9 KB | ||
| Filedesc metadata |  emd-24953.cif.gz emd-24953.cif.gz | 5.2 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-24953 http://ftp.pdbj.org/pub/emdb/structures/EMD-24953 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24953 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24953 | HTTPS FTP |
-Validation report
| Summary document |  emd_24953_validation.pdf.gz emd_24953_validation.pdf.gz | 440.5 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_24953_full_validation.pdf.gz emd_24953_full_validation.pdf.gz | 440 KB | Display | |
| Data in XML |  emd_24953_validation.xml.gz emd_24953_validation.xml.gz | 4.4 KB | Display | |
| Data in CIF |  emd_24953_validation.cif.gz emd_24953_validation.cif.gz | 4.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24953 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24953 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24953 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24953 | HTTPS FTP |
-Related structure data
| Related structure data |  7saqMC  7sarC  7sasC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-11041 (Title: Cryo electron-microscopy of TMEM106B fibrils extracted from four FTLD-TDP patient brains EMPIAR-11041 (Title: Cryo electron-microscopy of TMEM106B fibrils extracted from four FTLD-TDP patient brainsData size: 3.4 TB Data #1: Unaligned multi-frame micrographs of TMEM106B amyloid fibrils extracted from FTLD-TDP donor 1 [micrographs - multiframe] Data #2: Unaligned multi-frame micrographs of TMEM106B amyloid fibrils extracted from FTLD-TDP donor 2 [micrographs - multiframe] Data #3: Unaligned multi-frame micrographs of TMEM106B amyloid fibrils extracted from FTLD-TDP donor 3 [micrographs - multiframe] Data #4: Unaligned multi-frame micrographs of TMEM106B amyloid fibrils extracted from FTLD-TDP donor 4 [micrographs - multiframe]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_24953.map.gz / Format: CCP4 / Size: 7.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_24953.map.gz / Format: CCP4 / Size: 7.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. generated in cubic-lattice coordinate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.1 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : TMEM106B fibrils, polymorph 1
| Entire | Name: TMEM106B fibrils, polymorph 1 |
|---|---|
| Components |
|
-Supramolecule #1: TMEM106B fibrils, polymorph 1
| Supramolecule | Name: TMEM106B fibrils, polymorph 1 / type: organelle_or_cellular_component / ID: 1 / Parent: 0 / Macromolecule list: #1 Details: Amyloid fibrils extracted from autopsied brain tissues of a donor with FTLD-TDP |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Transmembrane protein 106B
| Macromolecule | Name: Transmembrane protein 106B / type: protein_or_peptide / ID: 1 Details: Glycosylation of Asn145, Asn151, Asn164, and Asn183 Number of copies: 5 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 31.156318 KDa |
| Sequence | String: MGKSLSHLPL HSSKEDAYDG VTSENMRNGL VNSEVHNEDG RNGDVSQFPY VEFTGRDSVT CPTCQGTGRI PRGQENQLVA LIPYSDQRL RPRRTKLYVM ASVFVCLLLS GLAVFFLFPR SIDVKYIGVK SAYVSYDVQK RTIYLNITNT LNITNNNYYS V EVENITAQ ...String: MGKSLSHLPL HSSKEDAYDG VTSENMRNGL VNSEVHNEDG RNGDVSQFPY VEFTGRDSVT CPTCQGTGRI PRGQENQLVA LIPYSDQRL RPRRTKLYVM ASVFVCLLLS GLAVFFLFPR SIDVKYIGVK SAYVSYDVQK RTIYLNITNT LNITNNNYYS V EVENITAQ VQFSKTVIGK ARLNNITIIG PLDMKQIDYT VPTVIAEEMS YMYDFCTLIS IKVHNIVLMM QVTVTTTYFG HS EQISQER YQYVDCGRNT TYQLGQSEYL NVLQPQQ UniProtKB: Transmembrane protein 106B |
-Macromolecule #2: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 2 / Number of copies: 20 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 298 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Detector mode: SUPER-RESOLUTION / Number grids imaged: 1 / Average electron dose: 39.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: AB INITIO MODEL / Overall B value: 124 |
|---|---|
| Output model |  PDB-7saq: |
 Movie
Movie Controller
Controller














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)