+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | SpCas9 bound to 8-nucleotide complementary DNA substrate | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | CRISPR / Cas9 / R-loop / substrate binding / off-target / HYDROLASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationmaintenance of CRISPR repeat elements / 3'-5' exonuclease activity / DNA endonuclease activity / defense response to virus / Hydrolases; Acting on ester bonds / DNA binding / RNA binding / metal ion binding Similarity search - Function | |||||||||
| Biological species |  Streptococcus pyogenes (bacteria) / synthetic construct (others) Streptococcus pyogenes (bacteria) / synthetic construct (others) | |||||||||
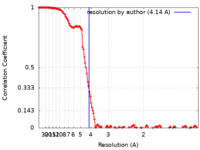
| Method | single particle reconstruction / cryo EM / Resolution: 4.14 Å | |||||||||
 Authors Authors | Pacesa M / Jinek M | |||||||||
| Funding support |  Switzerland, 1 items Switzerland, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2022 Journal: Nature / Year: 2022Title: R-loop formation and conformational activation mechanisms of Cas9. Authors: Martin Pacesa / Luuk Loeff / Irma Querques / Lena M Muckenfuss / Marta Sawicka / Martin Jinek /  Abstract: Cas9 is a CRISPR-associated endonuclease capable of RNA-guided, site-specific DNA cleavage. The programmable activity of Cas9 has been widely utilized for genome editing applications, yet its precise ...Cas9 is a CRISPR-associated endonuclease capable of RNA-guided, site-specific DNA cleavage. The programmable activity of Cas9 has been widely utilized for genome editing applications, yet its precise mechanisms of target DNA binding and off-target discrimination remain incompletely understood. Here we report a series of cryo-electron microscopy structures of Streptococcus pyogenes Cas9 capturing the directional process of target DNA hybridization. In the early phase of R-loop formation, the Cas9 REC2 and REC3 domains form a positively charged cleft that accommodates the distal end of the target DNA duplex. Guide-target hybridization past the seed region induces rearrangements of the REC2 and REC3 domains and relocation of the HNH nuclease domain to assume a catalytically incompetent checkpoint conformation. Completion of the guide-target heteroduplex triggers conformational activation of the HNH nuclease domain, enabled by distortion of the guide-target heteroduplex, and complementary REC2 and REC3 domain rearrangements. Together, these results establish a structural framework for target DNA-dependent activation of Cas9 that sheds light on its conformational checkpoint mechanism and may facilitate the development of novel Cas9 variants and guide RNA designs with enhanced specificity and activity. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_14494.map.gz emd_14494.map.gz | 106 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-14494-v30.xml emd-14494-v30.xml emd-14494.xml emd-14494.xml | 21.3 KB 21.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_14494_fsc.xml emd_14494_fsc.xml | 13.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_14494.png emd_14494.png | 54.6 KB | ||
| Filedesc metadata |  emd-14494.cif.gz emd-14494.cif.gz | 7.2 KB | ||
| Others |  emd_14494_half_map_1.map.gz emd_14494_half_map_1.map.gz emd_14494_half_map_2.map.gz emd_14494_half_map_2.map.gz | 200.4 MB 200.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-14494 http://ftp.pdbj.org/pub/emdb/structures/EMD-14494 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-14494 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-14494 | HTTPS FTP |
-Related structure data
| Related structure data |  7z4eMC  7z4cC  7z4dC  7z4gC  7z4hC  7z4iC  7z4jC  7z4kC  7z4lC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_14494.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_14494.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.65 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_14494_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_14494_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Ternary complex of SpCas9-sgRNA bound to a 8 nucleotide complemen...
| Entire | Name: Ternary complex of SpCas9-sgRNA bound to a 8 nucleotide complementary DNA substrate |
|---|---|
| Components |
|
-Supramolecule #1: Ternary complex of SpCas9-sgRNA bound to a 8 nucleotide complemen...
| Supramolecule | Name: Ternary complex of SpCas9-sgRNA bound to a 8 nucleotide complementary DNA substrate type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Streptococcus pyogenes (bacteria) Streptococcus pyogenes (bacteria) |
-Macromolecule #1: CRISPR-associated endonuclease Cas9/Csn1
| Macromolecule | Name: CRISPR-associated endonuclease Cas9/Csn1 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO / EC number: Hydrolases; Acting on ester bonds |
|---|---|
| Source (natural) | Organism:  Streptococcus pyogenes (bacteria) Streptococcus pyogenes (bacteria) |
| Molecular weight | Theoretical: 158.588781 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MDKKYSIGLA IGTNSVGWAV ITDEYKVPSK KFKVLGNTDR HSIKKNLIGA LLFDSGETAE ATRLKRTARR RYTRRKNRIC YLQEIFSNE MAKVDDSFFH RLEESFLVEE DKKHERHPIF GNIVDEVAYH EKYPTIYHLR KKLVDSTDKA DLRLIYLALA H MIKFRGHF ...String: MDKKYSIGLA IGTNSVGWAV ITDEYKVPSK KFKVLGNTDR HSIKKNLIGA LLFDSGETAE ATRLKRTARR RYTRRKNRIC YLQEIFSNE MAKVDDSFFH RLEESFLVEE DKKHERHPIF GNIVDEVAYH EKYPTIYHLR KKLVDSTDKA DLRLIYLALA H MIKFRGHF LIEGDLNPDN SDVDKLFIQL VQTYNQLFEE NPINASGVDA KAILSARLSK SRRLENLIAQ LPGEKKNGLF GN LIALSLG LTPNFKSNFD LAEDAKLQLS KDTYDDDLDN LLAQIGDQYA DLFLAAKNLS DAILLSDILR VNTEITKAPL SAS MIKRYD EHHQDLTLLK ALVRQQLPEK YKEIFFDQSK NGYAGYIDGG ASQEEFYKFI KPILEKMDGT EELLVKLNRE DLLR KQRTF DNGSIPHQIH LGELHAILRR QEDFYPFLKD NREKIEKILT FRIPYYVGPL ARGNSRFAWM TRKSEETITP WNFEE VVDK GASAQSFIER MTNFDKNLPN EKVLPKHSLL YEYFTVYNEL TKVKYVTEGM RKPAFLSGEQ KKAIVDLLFK TNRKVT VKQ LKEDYFKKIE CFDSVEISGV EDRFNASLGT YHDLLKIIKD KDFLDNEENE DILEDIVLTL TLFEDREMIE ERLKTYA HL FDDKVMKQLK RRRYTGWGRL SRKLINGIRD KQSGKTILDF LKSDGFANRN FMQLIHDDSL TFKEDIQKAQ VSGQGDSL H EHIANLAGSP AIKKGILQTV KVVDELVKVM GRHKPENIVI EMARENQTTQ KGQKNSRERM KRIEEGIKEL GSQILKEHP VENTQLQNEK LYLYYLQNGR DMYVDQELDI NRLSDYDVDA IVPQSFLKDD SIDNKVLTRS DKNRGKSDNV PSEEVVKKMK NYWRQLLNA KLITQRKFDN LTKAERGGLS ELDKAGFIKR QLVETRQITK HVAQILDSRM NTKYDENDKL IREVKVITLK S KLVSDFRK DFQFYKVREI NNYHHAHDAY LNAVVGTALI KKYPKLESEF VYGDYKVYDV RKMIAKSEQE IGKATAKYFF YS NIMNFFK TEITLANGEI RKRPLIETNG ETGEIVWDKG RDFATVRKVL SMPQVNIVKK TEVQTGGFSK ESILPKRNSD KLI ARKKDW DPKKYGGFDS PTVAYSVLVV AKVEKGKSKK LKSVKELLGI TIMERSSFEK NPIDFLEAKG YKEVKKDLII KLPK YSLFE LENGRKRMLA SAGELQKGNE LALPSKYVNF LYLASHYEKL KGSPEDNEQK QLFVEQHKHY LDEIIEQISE FSKRV ILAD ANLDKVLSAY NKHRDKPIRE QAENIIHLFT LTNLGAPAAF KYFDTTIDRK RYTSTKEVLD ATLIHQSITG LYETRI DLS QLGGD UniProtKB: CRISPR-associated endonuclease Cas9/Csn1 |
-Macromolecule #2: sgRNA
| Macromolecule | Name: sgRNA / type: rna / ID: 2 / Number of copies: 1 |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 33.005641 KDa |
| Sequence | String: GGGACGCAUA AAGAUGAGAC GCGUUUUAGA GCUAGAAAUA GCAAGUUAAA AUAAGGCUAG UCCGUUAUCA ACUUGAAAAA GUGGCACCG AGUCGGUGCU UUU |
-Macromolecule #3: Target strand of 8 nucleotide complementary DNA substrate
| Macromolecule | Name: Target strand of 8 nucleotide complementary DNA substrate type: dna / ID: 3 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 9.182928 KDa |
| Sequence | String: (DC)(DG)(DT)(DG)(DA)(DT)(DT)(DC)(DC)(DA) (DG)(DC)(DG)(DT)(DC)(DT)(DC)(DA)(DA)(DA) (DG)(DT)(DC)(DT)(DA)(DC)(DA)(DT)(DA) (DG) |
-Macromolecule #4: Non-target strand of 8 nucleotide complementary DNA substrate
| Macromolecule | Name: Non-target strand of 8 nucleotide complementary DNA substrate type: dna / ID: 4 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 9.211963 KDa |
| Sequence | String: (DC)(DT)(DA)(DT)(DG)(DT)(DA)(DG)(DA)(DC) (DT)(DT)(DA)(DC)(DT)(DA)(DT)(DT)(DA)(DT) (DT)(DG)(DG)(DA)(DA)(DT)(DC)(DA)(DC) (DG) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE-PROPANE / Chamber humidity: 100 % / Chamber temperature: 293.15 K |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 64.23 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.4 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)