[English] 日本語
 Yorodumi
Yorodumi- EMDB-11124: Cryo-electron tomogram after FIB-milling of Tuwongella immobilis,... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-11124 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
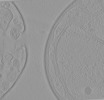
| Title | Cryo-electron tomogram after FIB-milling of Tuwongella immobilis, a species of the Planctomycetes phylum (high magnification, 33 000x) | ||||||||||||
 Map data Map data | Cryo-electron tomogram after FIB-milling of Tuwongella immobilis, a Planctomycetes species | ||||||||||||
 Sample Sample |
| ||||||||||||
| Biological species |  Tuwongella immobilis (bacteria) Tuwongella immobilis (bacteria) | ||||||||||||
| Method | electron tomography / cryo EM | ||||||||||||
 Authors Authors | Seeger C / Andersson SGE | ||||||||||||
| Funding support |  Sweden, 3 items Sweden, 3 items
| ||||||||||||
 Citation Citation |  Journal: Genome Biol Evol / Year: 2020 Journal: Genome Biol Evol / Year: 2020Title: Evolutionary Remodeling of the Cell Envelope in Bacteria of the Planctomycetes Phylum. Authors: Mayank Mahajan / Christian Seeger / Benjamin Yee / Siv G E Andersson /  Abstract: Bacteria of the Planctomycetes phylum have many unique cellular features, such as extensive membrane invaginations and the ability to import macromolecules. These features raise intriguing questions ...Bacteria of the Planctomycetes phylum have many unique cellular features, such as extensive membrane invaginations and the ability to import macromolecules. These features raise intriguing questions about the composition of their cell envelopes. In this study, we have used microscopy, phylogenomics, and proteomics to examine the composition and evolution of cell envelope proteins in Tuwongella immobilis and other members of the Planctomycetes. Cryo-electron tomography data indicated a distance of 45 nm between the inner and outer membranes in T. immobilis. Consistent with the wide periplasmic space, our bioinformatics studies showed that the periplasmic segments of outer-membrane proteins in type II secretion systems are extended in bacteria of the order Planctomycetales. Homologs of two highly abundant cysteine-rich cell wall proteins in T. immobilis were identified in all members of the Planctomycetales, whereas genes for peptidoglycan biosynthesis and cell elongation have been lost in many members of this bacterial group. The cell wall proteins contain multiple copies of the YTV motif, which is the only domain that is conserved and unique to the Planctomycetales. Earlier diverging taxa in the Planctomycetes phylum contain genes for peptidoglycan biosynthesis but no homologs to the YTV cell wall proteins. The major remodeling of the cell envelope in the ancestor of the Planctomycetales coincided with the emergence of budding and other unique cellular phenotypes. The results have implications for hypotheses about the process whereby complex cellular features evolve in bacteria. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_11124.map.gz emd_11124.map.gz | 1.1 GB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-11124-v30.xml emd-11124-v30.xml emd-11124.xml emd-11124.xml | 13 KB 13 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_11124.png emd_11124.png | 134.3 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-11124 http://ftp.pdbj.org/pub/emdb/structures/EMD-11124 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-11124 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-11124 | HTTPS FTP |
-Related structure data
| Related structure data | C: citing same article ( |
|---|---|
| EM raw data |  EMPIAR-10451 (Title: Cryo electron tomography after FIB-milling of Planctomycetes species Tuwongella immobilis (33k magnification) EMPIAR-10451 (Title: Cryo electron tomography after FIB-milling of Planctomycetes species Tuwongella immobilis (33k magnification)Data size: 15.5 Data #1: Single micrographs from cryo-ET tilt series (uncorrected) of Tuwongella immobilis after FIB-milling (33k magnification) [micrographs - single frame] Data #2: Single micrographs from cryo-ET tilt series (motion-corrected) of Tuwongella immobilis after FIB-milling (33k magnification) [micrographs - single frame] Data #3: Image stack (motion-corrected) from cryo-ET tilt series of Tuwongella immobilis after FIB-milling (33k magnification) [tilt series]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_11124.map.gz / Format: CCP4 / Size: 5.2 GB / Type: IMAGE STORED AS SIGNED BYTE Download / File: emd_11124.map.gz / Format: CCP4 / Size: 5.2 GB / Type: IMAGE STORED AS SIGNED BYTE | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-electron tomogram after FIB-milling of Tuwongella immobilis, a Planctomycetes species | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 4.37 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Tuwongella immobilis, whole cell cryo-electron tomogram after FIB...
| Entire | Name: Tuwongella immobilis, whole cell cryo-electron tomogram after FIB-milling. |
|---|---|
| Components |
|
-Supramolecule #1: Tuwongella immobilis, whole cell cryo-electron tomogram after FIB...
| Supramolecule | Name: Tuwongella immobilis, whole cell cryo-electron tomogram after FIB-milling. type: cell / ID: 1 / Parent: 0 Details: Whole cell tomogram of Tuwongella immobilis revealing the invaginated cytoplasmic membrane, which is characteristic for many members of the phylum Planctomycetes. |
|---|---|
| Source (natural) | Organism:  Tuwongella immobilis (bacteria) Tuwongella immobilis (bacteria) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | electron tomography |
| Aggregation state | cell |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 / Component - Concentration: 10.0 mmol/L / Component - Name: Na-phosphate Details: 10 mM Na-phosphate buffer, pH 7.4, 0.2 microm filtered. DO NOT use salt or any other components that increase osmolarity! |
|---|---|
| Grid | Model: Quantifoil R2/1 / Material: GOLD / Mesh: 200 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 101.325 kPa |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 293 K / Instrument: FEI VITROBOT MARK IV Details: Blot time: 6s, blot force: -5, wait time: 15s, 293K, 100% humidity. |
| Details | Bacterial cells grown on M1 agar plates at 32degrees celsius and kept at 20degress celsius until vitrification |
| Cryo protectant | No |
| Sectioning | Other: NO SECTIONING |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Temperature | Min: 93.0 K |
| Specialist optics | Energy filter - Slit width: 20 eV |
| Details | Before tilt series acquisition, cryo focused ion beam (FIB) milling with gallium ion source was performed on a FEI Dual beam Scios (Thermo Fisher Scientific, Netherlands). Autogrids with vitrified cells were mounted onto a dedicated cryo holder and transferred onto a cooled (93K) cryo stage in the dual beam microscope. The lamellae were prepared with two parallel rectangular patterns at both sides (top and bottom) of the cells, at milling angle of 10-15deg, accelerating voltage of 30 kV and ion currents between 30-300 pA. The final thickness of the lamella (milled at the lowest ion current) was about 150-300 nm. |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Number real images: 61 / Average electron dose: 1.64 e/Å2 Details: Total dose tilt series: 100e-/angstrom; number of tilts: 61; dose per tilt: 1.64 e-/angstrom: motion correction for the acquired tilt series was performed by using motioncor2 of the IMOD package |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Calibrated magnification: 33000 / Illumination mode: OTHER / Imaging mode: OTHER / Nominal magnification: 33000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Algorithm: BACK PROJECTION / Software - Name:  IMOD (ver. 4.9.10) / Number images used: 61 IMOD (ver. 4.9.10) / Number images used: 61 |
|---|
 Movie
Movie Controller
Controller