[English] 日本語
 Yorodumi
Yorodumi- EMDB-9717: Structure of the human GluN1/GluN2A NMDA receptor in the glutamat... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-9717 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of the human GluN1/GluN2A NMDA receptor in the glutamate/glycine-bound state at pH 6.3, Class III | ||||||||||||||||||
 Map data Map data | |||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||
 Keywords Keywords | ionotropic glutamate receptors / NMDA receptors / synaptic protein / MEMBRANE PROTEIN | ||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationglycine-gated cation channel activity / excitatory chemical synaptic transmission / directional locomotion / Synaptic adhesion-like molecules / protein localization to postsynaptic membrane / serotonin metabolic process / sleep / propylene metabolic process / response to glycine / Assembly and cell surface presentation of NMDA receptors ...glycine-gated cation channel activity / excitatory chemical synaptic transmission / directional locomotion / Synaptic adhesion-like molecules / protein localization to postsynaptic membrane / serotonin metabolic process / sleep / propylene metabolic process / response to glycine / Assembly and cell surface presentation of NMDA receptors / regulation of monoatomic cation transmembrane transport / Neurexins and neuroligins / NMDA glutamate receptor activity / NMDA selective glutamate receptor complex / glutamate binding / neurotransmitter receptor complex / ligand-gated sodium channel activity / glutamate receptor signaling pathway / calcium ion transmembrane import into cytosol / protein heterotetramerization / glycine binding / startle response / ligand-gated monoatomic ion channel activity / dopamine metabolic process / positive regulation of reactive oxygen species biosynthetic process / Negative regulation of NMDA receptor-mediated neuronal transmission / Unblocking of NMDA receptors, glutamate binding and activation / monoatomic cation transmembrane transport / positive regulation of calcium ion transport into cytosol / Long-term potentiation / regulation of neuronal synaptic plasticity / monoatomic cation transport / positive regulation of synaptic transmission, glutamatergic / synaptic cleft / calcium ion homeostasis / MECP2 regulates neuronal receptors and channels / EPHB-mediated forward signaling / glutamate-gated calcium ion channel activity / neurogenesis / excitatory synapse / sensory perception of pain / ionotropic glutamate receptor signaling pathway / Ras activation upon Ca2+ influx through NMDA receptor / cytoplasmic vesicle membrane / synaptic membrane / sodium ion transmembrane transport / positive regulation of excitatory postsynaptic potential / response to amphetamine / protein catabolic process / synaptic transmission, glutamatergic / excitatory postsynaptic potential / regulation of membrane potential / transmitter-gated monoatomic ion channel activity involved in regulation of postsynaptic membrane potential / negative regulation of protein catabolic process / regulation of synaptic plasticity / brain development / visual learning / postsynaptic density membrane / response to wounding / calcium ion transmembrane transport / memory / terminal bouton / synaptic vesicle / long-term synaptic potentiation / signaling receptor activity / amyloid-beta binding / RAF/MAP kinase cascade / presynaptic membrane / dendritic spine / response to ethanol / chemical synaptic transmission / learning or memory / postsynaptic membrane / calmodulin binding / neuron projection / postsynaptic density / positive regulation of apoptotic process / response to xenobiotic stimulus / calcium ion binding / synapse / dendrite / endoplasmic reticulum membrane / protein-containing complex binding / glutamatergic synapse / cell surface / positive regulation of transcription by RNA polymerase II / zinc ion binding / plasma membrane / cytoplasm Similarity search - Function | ||||||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 7.8 Å | ||||||||||||||||||
 Authors Authors | Zhang J / Chang S | ||||||||||||||||||
| Funding support |  China, 5 items China, 5 items
| ||||||||||||||||||
 Citation Citation |  Journal: Cell Rep / Year: 2018 Journal: Cell Rep / Year: 2018Title: Structural Basis of the Proton Sensitivity of Human GluN1-GluN2A NMDA Receptors. Authors: Jin-Bao Zhang / Shenghai Chang / Pan Xu / Miao Miao / Hangjun Wu / Youyi Zhang / Tongtong Zhang / Han Wang / Jilin Zhang / Chun Xie / Nan Song / Cheng Luo / Xing Zhang / Shujia Zhu /  Abstract: N-methyl-D-aspartate (NMDA) receptors are critical for synaptic development and plasticity. While glutamate is the primary agonist, protons can modulate NMDA receptor activity at synapses during ...N-methyl-D-aspartate (NMDA) receptors are critical for synaptic development and plasticity. While glutamate is the primary agonist, protons can modulate NMDA receptor activity at synapses during vesicle exocytosis by mechanisms that are unknown. We used cryo-electron microscopy to solve the structures of the human GluN1-GluN2A NMDA receptor at pH 7.8 and pH 6.3. Our structures demonstrate that the proton sensor predominantly resides in the N-terminal domain (NTD) of the GluN2A subunit and reveal the allosteric coupling mechanism between the proton sensor and the channel gate. Under high-pH conditions, the GluN2A-NTD adopts an "open-and-twisted" conformation. However, upon protonation at the lower pH, the GluN2A-NTD transits from an open- to closed-cleft conformation, causing rearrangements between the tetrameric NTDs and agonist-binding domains. The conformational mobility observed in our structures (presumably from protonation) is supported by molecular dynamics simulation. Our findings reveal the structural mechanisms by which protons allosterically inhibit human GluN1-GluN2A receptor activity. | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_9717.map.gz emd_9717.map.gz | 47.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-9717-v30.xml emd-9717-v30.xml emd-9717.xml emd-9717.xml | 20.3 KB 20.3 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_9717.png emd_9717.png | 69.3 KB | ||
| Filedesc metadata |  emd-9717.cif.gz emd-9717.cif.gz | 7.5 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-9717 http://ftp.pdbj.org/pub/emdb/structures/EMD-9717 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-9717 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-9717 | HTTPS FTP |
-Related structure data
| Related structure data |  6irhMC  9714C  9715C  9716C  6iraC  6irfC  6irgC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_9717.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_9717.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.01 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
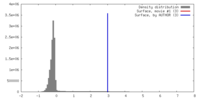
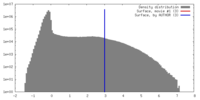
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Human GluN1/GluN2A NMDA receptors in the glutamate/glycine bound ...
| Entire | Name: Human GluN1/GluN2A NMDA receptors in the glutamate/glycine bound state at pH 6.3, Class III |
|---|---|
| Components |
|
-Supramolecule #1: Human GluN1/GluN2A NMDA receptors in the glutamate/glycine bound ...
| Supramolecule | Name: Human GluN1/GluN2A NMDA receptors in the glutamate/glycine bound state at pH 6.3, Class III type: complex / ID: 1 / Parent: 0 / Macromolecule list: all / Details: with the presence of Glycine,L-glutamate and EDTA |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 380 KDa |
-Macromolecule #1: Glutamate receptor ionotropic, NMDA 1
| Macromolecule | Name: Glutamate receptor ionotropic, NMDA 1 / type: protein_or_peptide / ID: 1 / Details: 2 mM Glycine / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 95.336219 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MSTMRLLTLA LLFSCSVARA ACDPKIVNIG AVLSTRKHEQ MFREAVNQAN KRHGSWKIQL NATSVTHKPN AIQMALSVCE DLISSQVYA ILVSHPPTPN DHFTPTPVSY TAGFYRIPVL GLTTRMSIYS DKSIHLSFLR TVPPYSHQSS VWFEMMRVYS W NHIILLVS ...String: MSTMRLLTLA LLFSCSVARA ACDPKIVNIG AVLSTRKHEQ MFREAVNQAN KRHGSWKIQL NATSVTHKPN AIQMALSVCE DLISSQVYA ILVSHPPTPN DHFTPTPVSY TAGFYRIPVL GLTTRMSIYS DKSIHLSFLR TVPPYSHQSS VWFEMMRVYS W NHIILLVS DDHEGRAAQK RLETLLEERE SKAEKVLQFD PGTKNVTALL MEAKELEARV IILSASEDDA ATVYRAAAML NM TGSGYVW LVGEREISGN ALRYAPDGIL GLQLINGKNE SAHISDAVGV VAQAVHELLE KENITDPPRG CVGNTNIWKT GPL FKRVLM SSKYADGVTG RVEFNEDGDR KFANYSIMNL QNRKLVQVGI YNGTHVIPND RKIIWPGGET EKPRGYQMST RLKI VTIHQ EPFVYVKPTL SDGTCKEEFT VNGDPVKKVI CTGPNDTSPG SPRHTVPQCC YGFCIDLLIK LARTMNFTYE VHLVA DGKF GTQERVNNSN KKEWNGMMGE LLSGQADMIV APLTINNERA QYIEFSKPFK YQGLTILVKK EIPRSTLDSF MQPFQS TLW LLVGLSVHVV AVMLYLLDRF SPFGRFKVNS EEEEEDALTL SSAMWFSWRV LLNSGIGEGA PRSFSARILG MVWAGFA MI IVASYTANLA AFLVLDRPEE RITGINDPRL RNPSDKFIYA TVKQSSVDIY FRRQVELSTM YRHMEKHNYE SAAEAIQA V RDNKLHAFIW DSAVLEFEAS QKCDLVTTGE LFFRSGFGIG MRKDSPWKQN VSLSILKSHE NGFMEDLDKT WVRYQECDS RSNAPATLTF ENMAGVFMLV AGGIVAGIFL IFIEIAYKRH KDARRKQ UniProtKB: Glutamate receptor ionotropic, NMDA 1 |
-Macromolecule #2: Glutamate receptor ionotropic, NMDA 2A
| Macromolecule | Name: Glutamate receptor ionotropic, NMDA 2A / type: protein_or_peptide / ID: 2 / Details: 2 mM L-Glutamate / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 94.192172 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MGRVGYWTLL VLPALLVWRG PAPSAAAEKG PPALNIAVML GHSHDVTERE LRTLWGPEQA AGLPLDVNVV ALLMNRTDPK SLITHVCDL MSGARIHGLV FGDDTDQEAV AQMLDFISSH TFVPILGIHG GASMIMADKD PTSTFFQFGA SIQQQATVML K IMQDYDWH ...String: MGRVGYWTLL VLPALLVWRG PAPSAAAEKG PPALNIAVML GHSHDVTERE LRTLWGPEQA AGLPLDVNVV ALLMNRTDPK SLITHVCDL MSGARIHGLV FGDDTDQEAV AQMLDFISSH TFVPILGIHG GASMIMADKD PTSTFFQFGA SIQQQATVML K IMQDYDWH VFSLVTTIFP GYREFISFVK TTVDNSFVGW DMQNVITLDT SFEDAKTQVQ LKKIHSSVIL LYCSKDEAVL IL SEARSLG LTGYDFFWIV PSLVSGNTEL IPKEFPSGLI SVSYDDWDYS LEARVRDGIG ILTTAASSML EKFSYIPEAK ASC YGQMER PEVPMHTLHP FMVNVTWDGK DLSFTEEGYQ VHPRLVVIVL NKDREWEKVG KWENHTLSLR HAVWPRYKSF SDCE PDDNH LSIVTLEEAP FVIVEDIDPL TETCVRNTVP CRKFVKINNS TNEGMNVKKC CKGFCIDILK KLSRTVKFTY DLYLV TNGK HGKKVNNVWN GMIGEVVYQR AVMAVGSLTI NEERSEVVDF SVPFVETGIS VMVSRSNGTV SPSAFLEPFS ASVWVM MFV MLLIVSAIAV FVFEYFSPVG YNRNLAKGKA PHGPSFTIGK AIWLLWGLVF NNSVPVQNPK GTTSKIMVSV WAFFAVI FL ASYTANLAAF MIQRRFVDQV TGLSDKKFQR PHDYSPPFRF GTVPNGSTER NIRNNYPYMH QYMTKFNQKG VEDALVSL K TGKLDAFIYD AAVLNYKAGR DEGCKLVTIG SGYIFATTGY GIALQKGSPW KRQIDLALLQ FVGDGEMEEL ETLWLTGIC HNEKNEVMSS QLDIDNMAGV FYMLAAAMAL SLITFIWEHL F UniProtKB: Glutamate receptor ionotropic, NMDA 2A |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 3.5 mg/mL | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 6.3 Component:
Details: Solutions were made fresh. | |||||||||||||||||||||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 200 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 45 sec. / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 260.0 kPa / Details: 15 mA | |||||||||||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 291 K / Instrument: FEI VITROBOT MARK II Details: blot for 2 seconds before plunging in liquid ethane. | |||||||||||||||||||||||||||
| Details | Tetrameric GluN1/GluN2A NMDA receptors |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Digitization - Frames/image: 1-40 / Number grids imaged: 4 / Average exposure time: 12.0 sec. / Average electron dose: 56.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model |
| ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Protocol: RIGID BODY FIT | ||||||||||||||||
| Output model |  PDB-6irh: |
 Movie
Movie Controller
Controller





















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)