[English] 日本語
 Yorodumi
Yorodumi- PDB-8uhb: Cryo-EM Structure of the Ro5256390-bound hTA1-Gs heterotrimer sig... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8uhb | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM Structure of the Ro5256390-bound hTA1-Gs heterotrimer signaling complex | |||||||||||||||
 Components Components |
| |||||||||||||||
 Keywords Keywords | MEMBRANE PROTEIN / GPCR / Signaling Complex / Trace Amine-associated Receptor | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationadenylate cyclase regulator activity / regulation of parathyroid hormone secretion / COPI-coated Golgi to ER transport vesicle / post-embryonic body morphogenesis / Amine ligand-binding receptors / : / response to parathyroid hormone / trace-amine receptor activity / genomic imprinting / positive regulation of cAMP-mediated signaling ...adenylate cyclase regulator activity / regulation of parathyroid hormone secretion / COPI-coated Golgi to ER transport vesicle / post-embryonic body morphogenesis / Amine ligand-binding receptors / : / response to parathyroid hormone / trace-amine receptor activity / genomic imprinting / positive regulation of cAMP-mediated signaling / sensory perception of chemical stimulus / tissue homeostasis / positive regulation of sodium ion transport / energy reserve metabolic process / endochondral ossification / positive regulation of osteoclast differentiation / embryonic cranial skeleton morphogenesis / : / beta2-adrenergic receptor activity / positive regulation of mini excitatory postsynaptic potential / embryonic hindlimb morphogenesis / cartilage development / positive regulation of cAMP-dependent protein kinase activity / norepinephrine binding / alkylglycerophosphoethanolamine phosphodiesterase activity / Adrenoceptors / heat generation / positive regulation of autophagosome maturation / positive regulation of AMPA receptor activity / norepinephrine-epinephrine-mediated vasodilation involved in regulation of systemic arterial blood pressure / activation of transmembrane receptor protein tyrosine kinase activity / negative regulation of smooth muscle contraction / positive regulation of lipophagy / skin development / response to psychosocial stress / negative regulation of multicellular organism growth / adrenergic receptor signaling pathway / endosome to lysosome transport / cellular response to glucagon stimulus / diet induced thermogenesis / neuronal dense core vesicle / positive regulation of protein kinase A signaling / hair follicle placode formation / adenylate cyclase binding / smooth muscle contraction / alpha-tubulin binding / mu-type opioid receptor binding / developmental growth / corticotropin-releasing hormone receptor 1 binding / G-protein alpha-subunit binding / regulation of signal transduction / D1 dopamine receptor binding / potassium channel regulator activity / positive regulation of bone mineralization / positive regulation of osteoblast differentiation / brown fat cell differentiation / beta-2 adrenergic receptor binding / adenylate cyclase-activating adrenergic receptor signaling pathway / regulation of sodium ion transport / bone resorption / ruffle / activation of adenylate cyclase activity / adenylate cyclase activator activity / negative regulation of blood pressure / response to cold / receptor-mediated endocytosis / post-embryonic development / G protein activity / positive regulation of GTPase activity / trans-Golgi network membrane / skeletal system development / G protein-coupled receptor activity / electron transport chain / ionotropic glutamate receptor binding / insulin-like growth factor receptor binding / clathrin-coated endocytic vesicle membrane / G-protein beta/gamma-subunit complex binding / Olfactory Signaling Pathway / bone development / Activation of the phototransduction cascade / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / positive regulation of protein serine/threonine kinase activity / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / multicellular organism growth / G-protein activation / adenylate cyclase-activating G protein-coupled receptor signaling pathway / positive regulation of insulin secretion / G protein-coupled acetylcholine receptor signaling pathway / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / Prostacyclin signalling through prostacyclin receptor / recycling endosome / Glucagon signaling in metabolic regulation / G beta:gamma signalling through CDC42 / cognition / ADP signalling through P2Y purinoceptor 12 / G beta:gamma signalling through BTK / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) Similarity search - Function | |||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human)  | |||||||||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.35 Å | |||||||||||||||
 Authors Authors | Zilberg, G. / Warren, A.L. / Parpounas, A.K. / Wacker, D. | |||||||||||||||
| Funding support |  United States, 4items United States, 4items
| |||||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Molecular basis of human trace amine-associated receptor 1 activation. Authors: Gregory Zilberg / Alexandra K Parpounas / Audrey L Warren / Shifan Yang / Daniel Wacker /  Abstract: The human trace amine-associated receptor 1 (hTAAR1, hTA1) is a key regulator of monoaminergic neurotransmission and the actions of psychostimulants. Despite preclinical research demonstrating its ...The human trace amine-associated receptor 1 (hTAAR1, hTA1) is a key regulator of monoaminergic neurotransmission and the actions of psychostimulants. Despite preclinical research demonstrating its tractability as a drug target, its molecular mechanisms of activation remain unclear. Moreover, poorly understood pharmacological differences between rodent and human TA1 complicate the translation of findings from preclinical disease models into novel pharmacotherapies. To elucidate hTA1's mechanisms on the molecular scale and investigate the underpinnings of its divergent pharmacology from rodent orthologs, we herein report the structure of the human TA1 receptor in complex with a Gαs heterotrimer. Our structure reveals shared structural elements with other TAARs, as well as with its closest monoaminergic orthologue, the serotonin receptor 5-HT4R. We further find that a single mutation dramatically shifts the selectivity of hTA1 towards that of its rodent orthologues, and report on the effects of substituting residues to those found in serotonin and dopamine receptors. Strikingly, we also discover that the atypical antipsychotic medication and pan-monoaminergic antagonist asenapine potently and efficaciously activates hTA1. Together our studies provide detailed insight into hTA1 structure and function, contrast its molecular pharmacology with that of related receptors, and uncover off-target activities of monoaminergic drugs at hTA1. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8uhb.cif.gz 8uhb.cif.gz | 364 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8uhb.ent.gz pdb8uhb.ent.gz | 291.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8uhb.json.gz 8uhb.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  8uhb_validation.pdf.gz 8uhb_validation.pdf.gz | 1.1 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  8uhb_full_validation.pdf.gz 8uhb_full_validation.pdf.gz | 1.1 MB | Display | |
| Data in XML |  8uhb_validation.xml.gz 8uhb_validation.xml.gz | 40.2 KB | Display | |
| Data in CIF |  8uhb_validation.cif.gz 8uhb_validation.cif.gz | 59.2 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/uh/8uhb https://data.pdbj.org/pub/pdb/validation_reports/uh/8uhb ftp://data.pdbj.org/pub/pdb/validation_reports/uh/8uhb ftp://data.pdbj.org/pub/pdb/validation_reports/uh/8uhb | HTTPS FTP |
-Related structure data
| Related structure data | 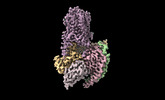 42268MC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Guanine nucleotide-binding protein ... , 3 types, 3 molecules BCG
| #2: Protein | Mass: 39418.086 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: GNB1 / Cell line (production host): Sf9 / Production host: Homo sapiens (human) / Gene: GNB1 / Cell line (production host): Sf9 / Production host:  |
|---|---|
| #3: Protein | Mass: 45803.598 Da / Num. of mol.: 1 Mutation: E189D, M191V, T193S, E194D, N271K, K274D, R280K, T284D, I285T, G226A, A366S Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
| #4: Protein | Mass: 7861.143 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: GNG2 / Production host: Homo sapiens (human) / Gene: GNG2 / Production host:  |
-Protein / Antibody / Non-polymers , 3 types, 3 molecules AN

| #1: Protein | Mass: 60019.816 Da / Num. of mol.: 1 / Mutation: M(-129)W, R(-14)G, Q(-3)E, F112W Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: cybC, ADRB2, TAAR1 / Cell line (production host): Sf9 / Production host: Homo sapiens (human) / Gene: cybC, ADRB2, TAAR1 / Cell line (production host): Sf9 / Production host:  References: UniProt: P0ABE7, UniProt: P07550, UniProt: Q96RJ0 |
|---|---|
| #5: Antibody | Mass: 14714.320 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
| #6: Chemical | ChemComp-WV8 / ( |
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: hTA1-Gs-Nb35 complex (Ro5256390) / Type: COMPLEX / Entity ID: #1-#5 / Source: RECOMBINANT |
|---|---|
| Molecular weight | Units: MEGADALTONS / Experimental value: NO |
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Source (recombinant) | Organism:  |
| Buffer solution | pH: 7.4 |
| Specimen | Conc.: 1.2 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Specimen support | Grid material: COPPER / Grid mesh size: 300 divisions/in. / Grid type: Quantifoil R1.2/1.3 |
| Vitrification | Instrument: LEICA EM GP / Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal defocus max: 1800 nm / Nominal defocus min: 500 nm |
| Image recording | Electron dose: 53.88 e/Å2 / Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) |
- Processing
Processing
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3D reconstruction | Resolution: 3.35 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 626370 / Symmetry type: POINT | ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller


 PDBj
PDBj



























