+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-8639 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM map of Hrd1 dimer with one Hrd3 molecule | |||||||||
 Map data Map data | Hrd1 dimer with one Hrd3 binding map with low-pass filtering to 4.7A and applied -250 b-factor | |||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationHrd1p ubiquitin ligase ERAD-M complex / detection of unfolded protein / luminal surveillance complex / Hrd1p ubiquitin ligase complex / Hrd1p ubiquitin ligase ERAD-L complex / negative regulation of protein autoubiquitination / retrograde protein transport, ER to cytosol / ERAD pathway / endoplasmic reticulum membrane / endoplasmic reticulum Similarity search - Function | |||||||||
| Biological species |  | |||||||||
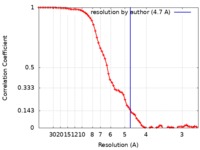
| Method | single particle reconstruction / cryo EM / Resolution: 4.7 Å | |||||||||
 Authors Authors | Schoebel S / Mi W / Stein A / Rapoport TA / Liao M | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2017 Journal: Nature / Year: 2017Title: Cryo-EM structure of the protein-conducting ERAD channel Hrd1 in complex with Hrd3. Authors: Stefan Schoebel / Wei Mi / Alexander Stein / Sergey Ovchinnikov / Ryan Pavlovicz / Frank DiMaio / David Baker / Melissa G Chambers / Huayou Su / Dongsheng Li / Tom A Rapoport / Maofu Liao /    Abstract: Misfolded endoplasmic reticulum proteins are retro-translocated through the membrane into the cytosol, where they are poly-ubiquitinated, extracted from the membrane, and degraded by the proteasome-a ...Misfolded endoplasmic reticulum proteins are retro-translocated through the membrane into the cytosol, where they are poly-ubiquitinated, extracted from the membrane, and degraded by the proteasome-a pathway termed endoplasmic reticulum-associated protein degradation (ERAD). Proteins with misfolded domains in the endoplasmic reticulum lumen or membrane are discarded through the ERAD-L and ERAD-M pathways, respectively. In Saccharomyces cerevisiae, both pathways require the ubiquitin ligase Hrd1, a multi-spanning membrane protein with a cytosolic RING finger domain. Hrd1 is the crucial membrane component for retro-translocation, but it is unclear whether it forms a protein-conducting channel. Here we present a cryo-electron microscopy structure of S. cerevisiae Hrd1 in complex with its endoplasmic reticulum luminal binding partner, Hrd3. Hrd1 forms a dimer within the membrane with one or two Hrd3 molecules associated at its luminal side. Each Hrd1 molecule has eight transmembrane segments, five of which form an aqueous cavity extending from the cytosol almost to the endoplasmic reticulum lumen, while a segment of the neighbouring Hrd1 molecule forms a lateral seal. The aqueous cavity and lateral gate are reminiscent of features of protein-conducting conduits that facilitate polypeptide movement in the opposite direction-from the cytosol into or across membranes. Our results suggest that Hrd1 forms a retro-translocation channel for the movement of misfolded polypeptides through the endoplasmic reticulum membrane. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_8639.map.gz emd_8639.map.gz | 25 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-8639-v30.xml emd-8639-v30.xml emd-8639.xml emd-8639.xml | 14.6 KB 14.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_8639_fsc.xml emd_8639_fsc.xml | 8 KB | Display |  FSC data file FSC data file |
| Images |  emd_8639.png emd_8639.png | 96.7 KB | ||
| Others |  emd_8639_additional.map.gz emd_8639_additional.map.gz | 20.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-8639 http://ftp.pdbj.org/pub/emdb/structures/EMD-8639 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8639 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8639 | HTTPS FTP |
-Validation report
| Summary document |  emd_8639_validation.pdf.gz emd_8639_validation.pdf.gz | 505.3 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_8639_full_validation.pdf.gz emd_8639_full_validation.pdf.gz | 504.8 KB | Display | |
| Data in XML |  emd_8639_validation.xml.gz emd_8639_validation.xml.gz | 9.5 KB | Display | |
| Data in CIF |  emd_8639_validation.cif.gz emd_8639_validation.cif.gz | 12.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8639 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8639 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8639 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8639 | HTTPS FTP |
-Related structure data
| Related structure data |  8637C  8638C  8642C  5v6pC  5v7vC C: citing same article ( |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10099 (Title: Cryo-EM structure of Hrd1 and Hrd3 complex / Data size: 708.3 EMPIAR-10099 (Title: Cryo-EM structure of Hrd1 and Hrd3 complex / Data size: 708.3 Data #1: summed frame micrographs of Hrd1/Hrd3 complex [micrographs - single frame] Data #2: dose-weighted summed frame micrographs of Hrd1/Hrd3 complex [micrographs - single frame] Data #3: picked particles of Hrd1/Hrd3 complex [picked particles - multiframe - processed]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_8639.map.gz / Format: CCP4 / Size: 27 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_8639.map.gz / Format: CCP4 / Size: 27 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Hrd1 dimer with one Hrd3 binding map with low-pass filtering to 4.7A and applied -250 b-factor | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
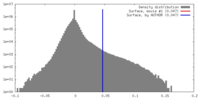
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.35 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: Hrd1 dimer with one Hrd3 binding map without...
| File | emd_8639_additional.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Hrd1 dimer with one Hrd3 binding map without filtering or amplitude modification | ||||||||||||
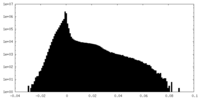
| Projections & Slices |
| ||||||||||||
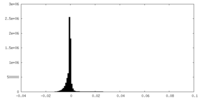
| Density Histograms |
- Sample components
Sample components
-Entire : Hrd1 dimer with one Hrd3 molecule
| Entire | Name: Hrd1 dimer with one Hrd3 molecule |
|---|---|
| Components |
|
-Supramolecule #1: Hrd1 dimer with one Hrd3 molecule
| Supramolecule | Name: Hrd1 dimer with one Hrd3 molecule / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
| Recombinant expression | Organism:  |
-Macromolecule #1: ERAD-associated E3 ubiquitin-protein ligase HRD1
| Macromolecule | Name: ERAD-associated E3 ubiquitin-protein ligase HRD1 / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Recombinant expression | Organism:  |
| Sequence | String: MVPENRRKQL AIFVVVTYLL TFYCVYSATK TSVSFLQVTL KLNEGFNLMV LSIFILLNST LL WQLLTKL LFGELRLIEH EHIFERLPFT IINTLFMSSL FHERYFFTVA FFGLLLLYLK VFHWILKDRL EALLQSINDS TTMKTLIFSR FSFNLV LLA VVDYQIITRC ...String: MVPENRRKQL AIFVVVTYLL TFYCVYSATK TSVSFLQVTL KLNEGFNLMV LSIFILLNST LL WQLLTKL LFGELRLIEH EHIFERLPFT IINTLFMSSL FHERYFFTVA FFGLLLLYLK VFHWILKDRL EALLQSINDS TTMKTLIFSR FSFNLV LLA VVDYQIITRC ISSIYTNQKS DIESTSLYLI QVMEFTMLLI DLLNLFLQTC LNFWEFYRSQ QSLSNENNHI VHGDPTDENT VESDQSQPVL NDDDDDDDD DRQFTGLEGK FMYEKAIDVF TRFLKTALHL SMLIPFRMPM MLLKDVVWDI LALYQSGTSL WKIWRNNKQL DDTLVTVTVE QLQN SANDD NICIICMDEL IHSPNQQTWK NKNKKPKRLP CGHILHLSCL KNWMERSQTC PICRLPVFDE K |
-Macromolecule #2: ERAD-associated E3 ubiquitin-protein ligase component HRD3
| Macromolecule | Name: ERAD-associated E3 ubiquitin-protein ligase component HRD3 type: protein_or_peptide / ID: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Recombinant expression | Organism:  |
| Sequence | String: MITLLLYLCV ICNAIVLIRA DSIADPWPEA RHLLNTIAKS RDPMKEAAME PNADEFVGFY VPMDYSPRN EEKNYQSIWQ NEITDSQRHI YELLVQSSEQ FNNSEATYTL SQIHLWSQYN F PHNMTLAH KYLEKFNDLT HFTNHSAIFD LAVMYATGGC ASGNDQTVIP ...String: MITLLLYLCV ICNAIVLIRA DSIADPWPEA RHLLNTIAKS RDPMKEAAME PNADEFVGFY VPMDYSPRN EEKNYQSIWQ NEITDSQRHI YELLVQSSEQ FNNSEATYTL SQIHLWSQYN F PHNMTLAH KYLEKFNDLT HFTNHSAIFD LAVMYATGGC ASGNDQTVIP QDSAKALLYY QR AAQLGNL KAKQVLAYKY YSGFNVPRNF HKSLVLYRDI AEQLRKSYSR DEWDIVFPYW ESY NVRISD FESGLLGKGL NSVPSSTVRK RTTRPDIGSP FIAQVNGVQM TLQIEPMGRF AFNG NDGNI NGDEDDEDAS ERRIIRIYYA ALNDYKGTYS QSRNCERAKN LLELTYKEFQ PHVDN LDPL QVFYYVRCLQ LLGHMYFTGE GSSKPNIHMA EEILTTSLEI SRRAQGPIGR ACIDLG LIN QYITNNISQA ISYYMKAMKT QANNGIVEFQ LSKLATSFPE EKIGDPFNLM ETAYLNG FI PAIYEFAVMI ESGMNSKSSV ENTAYLFKTF VDKNEAIMAP KLRTAFAALI NDRSEVAL W AYSQLAEQGY ETAQVSAAYL MYQLPYEFED PPRTTDQRKT LAISYYTRAF KQGNIDAGV VAGDIYFQMQ NYSKAMALYQ GAALKYSIQA IWNLGYMHEH GLGVNRDFHL AKRYYDQVSE HDHRFYLAS KLSVLKLHLK SWLTWITREK VNYWKPSSPL NPNEDTQHSK TSWYKQLTKI L QRMRHKED SDKAAEDSHK HRTVVQNGAN HRGDDQEEAS EILGFQMEDG GGENLYFQSG GG MDEKTTG WRGGHVVEGL AGELEQLRAR LEHHPQGQRE P |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.8 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 400 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Atmosphere: AIR |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 82.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)