+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7ey9 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | tail proteins | ||||||||||||
 Components Components |
| ||||||||||||
 Keywords Keywords | VIRAL PROTEIN / bacteriophae T7 tail proteins / VIRUS | ||||||||||||
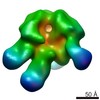
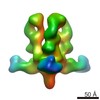
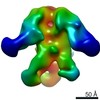
| Function / homology |  Function and homology information Function and homology informationvirus tail, tube / symbiont genome ejection through host cell envelope, short tail mechanism / virus tail, fiber / adhesion receptor-mediated virion attachment to host cell / symbiont entry into host cell / virion attachment to host cell / identical protein binding Similarity search - Function | ||||||||||||
| Biological species |   Escherichia phage T7 (virus) Escherichia phage T7 (virus) | ||||||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.4 Å | ||||||||||||
 Authors Authors | Liu, H.R. / Chen, W.Y. | ||||||||||||
| Funding support |  China, 3items China, 3items
| ||||||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2021 Journal: Proc Natl Acad Sci U S A / Year: 2021Title: Structural changes in bacteriophage T7 upon receptor-induced genome ejection. Authors: Wenyuan Chen / Hao Xiao / Li Wang / Xurong Wang / Zhixue Tan / Zhen Han / Xiaowu Li / Fan Yang / Zhonghua Liu / Jingdong Song / Hongrong Liu / Lingpeng Cheng /  Abstract: Many tailed bacteriophages assemble ejection proteins and a portal-tail complex at a unique vertex of the capsid. The ejection proteins form a transenvelope channel extending the portal-tail channel ...Many tailed bacteriophages assemble ejection proteins and a portal-tail complex at a unique vertex of the capsid. The ejection proteins form a transenvelope channel extending the portal-tail channel for the delivery of genomic DNA in cell infection. Here, we report the structure of the mature bacteriophage T7, including the ejection proteins, as well as the structures of the full and empty T7 particles in complex with their cell receptor lipopolysaccharide. Our near-atomic-resolution reconstruction shows that the ejection proteins in the mature T7 assemble into a core, which comprises a fourfold gene product 16 (gp16) ring, an eightfold gp15 ring, and a putative eightfold gp14 ring. The gp15 and gp16 are mainly composed of helix bundles, and gp16 harbors a lytic transglycosylase domain for degrading the bacterial peptidoglycan layer. When interacting with the lipopolysaccharide, the T7 tail nozzle opens. Six copies of gp14 anchor to the tail nozzle, extending the nozzle across the lipopolysaccharide lipid bilayer. The structures of gp15 and gp16 in the mature T7 suggest that they should undergo remarkable conformational changes to form the transenvelope channel. Hydrophobic α-helices were observed in gp16 but not in gp15, suggesting that gp15 forms the channel in the hydrophilic periplasm and gp16 forms the channel in the cytoplasmic membrane. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7ey9.cif.gz 7ey9.cif.gz | 1.6 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7ey9.ent.gz pdb7ey9.ent.gz | 1.3 MB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7ey9.json.gz 7ey9.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  7ey9_validation.pdf.gz 7ey9_validation.pdf.gz | 1.1 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  7ey9_full_validation.pdf.gz 7ey9_full_validation.pdf.gz | 1.2 MB | Display | |
| Data in XML |  7ey9_validation.xml.gz 7ey9_validation.xml.gz | 204.8 KB | Display | |
| Data in CIF |  7ey9_validation.cif.gz 7ey9_validation.cif.gz | 334.2 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ey/7ey9 https://data.pdbj.org/pub/pdb/validation_reports/ey/7ey9 ftp://data.pdbj.org/pub/pdb/validation_reports/ey/7ey9 ftp://data.pdbj.org/pub/pdb/validation_reports/ey/7ey9 | HTTPS FTP |
-Related structure data
| Related structure data |  31319MC  7ey6C  7ey7C  7ey8C  7eybC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
| #1: Protein | Mass: 61641.875 Da / Num. of mol.: 18 / Source method: isolated from a natural source / Source: (natural)   Escherichia phage T7 (virus) / References: UniProt: P03748 Escherichia phage T7 (virus) / References: UniProt: P03748#2: Protein | Mass: 89480.594 Da / Num. of mol.: 6 / Source method: isolated from a natural source / Source: (natural)   Escherichia phage T7 (virus) / References: UniProt: P03747 Escherichia phage T7 (virus) / References: UniProt: P03747#3: Protein | Mass: 22307.650 Da / Num. of mol.: 12 / Source method: isolated from a natural source / Source: (natural)   Escherichia phage T7 (virus) / References: UniProt: P03746 Escherichia phage T7 (virus) / References: UniProt: P03746 |
|---|
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Escherichia phage T7 / Type: COMPLEX / Entity ID: all / Source: NATURAL |
|---|---|
| Source (natural) | Organism:   Escherichia phage T7 (virus) Escherichia phage T7 (virus) |
| Details of virus | Empty: NO / Enveloped: NO / Isolate: SPECIES / Type: VIRUS-LIKE PARTICLE |
| Buffer solution | pH: 7.4 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TECNAI ARCTICA |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 200 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 200 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD |
| Image recording | Electron dose: 25 e/Å2 / Film or detector model: FEI FALCON II (4k x 4k) |
- Processing
Processing
| CTF correction | Type: NONE |
|---|---|
| Symmetry | Point symmetry: C6 (6 fold cyclic) |
| 3D reconstruction | Resolution: 3.4 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 75068 / Symmetry type: POINT |
 Movie
Movie Controller
Controller













 PDBj
PDBj