+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7scf | ||||||
|---|---|---|---|---|---|---|---|
| Title | M. tb EgtD in complex with HD2 | ||||||
 Components Components | Histidine N-alpha-methyltransferase | ||||||
 Keywords Keywords | TRANSFERASE / Ergothioneine biosynthesis pathway / Rossmann fold domain / histidine/histamine derivatives / SAM dependent methyltransferase | ||||||
| Function / homology |  Function and homology information Function and homology informationergothioneine biosynthetic process / L-histidine Nalpha-methyltransferase / L-histidine N(alpha)-methyltransferase activity / protein methyltransferase activity / methylation Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.67 Å MOLECULAR REPLACEMENT / Resolution: 2.67 Å | ||||||
 Authors Authors | Sudasinghe, T.D. / Ronning, D.R. | ||||||
| Funding support |  United States, 1items United States, 1items
| ||||||
 Citation Citation |  Journal: Sci Rep / Year: 2021 Journal: Sci Rep / Year: 2021Title: Inhibitors of Mycobacterium tuberculosis EgtD target both substrate binding sites to limit hercynine production. Authors: Sudasinghe, T.D. / Banco, M.T. / Ronning, D.R. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7scf.cif.gz 7scf.cif.gz | 253.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7scf.ent.gz pdb7scf.ent.gz | 204 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7scf.json.gz 7scf.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/sc/7scf https://data.pdbj.org/pub/pdb/validation_reports/sc/7scf ftp://data.pdbj.org/pub/pdb/validation_reports/sc/7scf ftp://data.pdbj.org/pub/pdb/validation_reports/sc/7scf | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  7sewC  7sexC  7seyC  7sf4C  7sf5C  4uy5S S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 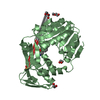
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 2 molecules AB
| #1: Protein | Mass: 35314.789 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Gene: egtD, C0094_20235, DSI38_25660, E5M05_18135, E5M52_16355, E5M78_15990, ERS007657_01802, ERS007663_00740, ERS007665_01629, ERS007670_03296, ERS007741_03430, ERS013471_00673, ERS024276_01805, ...Gene: egtD, C0094_20235, DSI38_25660, E5M05_18135, E5M52_16355, E5M78_15990, ERS007657_01802, ERS007663_00740, ERS007665_01629, ERS007670_03296, ERS007741_03430, ERS013471_00673, ERS024276_01805, ERS094182_02516, F6W99_02380, GCL30_14140, SAMEA2683035_00801 Production host:  References: UniProt: A0A045KE74, L-histidine Nalpha-methyltransferase |
|---|
-Non-polymers , 5 types, 105 molecules 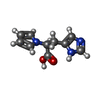








| #2: Chemical | | #3: Chemical | ChemComp-PEG / #4: Chemical | ChemComp-GOL / | #5: Chemical | #6: Water | ChemComp-HOH / | |
|---|
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.54 Å3/Da / Density % sol: 51.57 % |
|---|---|
| Crystal grow | Temperature: 298.15 K / Method: vapor diffusion, hanging drop Details: 0.2 M potassium phosphate dibasic and 20 % w/v polyethylene glycol 3,350 |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 21-ID-D / Wavelength: 0.98 Å / Beamline: 21-ID-D / Wavelength: 0.98 Å |
| Detector | Type: MARMOSAIC 225 mm CCD / Detector: CCD / Date: Jul 31, 2020 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.98 Å / Relative weight: 1 |
| Reflection | Resolution: 2.67→50.95 Å / Num. obs: 21089 / % possible obs: 99.84 % / Redundancy: 1 % / CC1/2: 0.96 / Rmerge(I) obs: 0.1 / Net I/σ(I): 10.89 |
| Reflection shell | Resolution: 2.67→2.67 Å / Rmerge(I) obs: 0.45 / Num. unique obs: 2068 / CC1/2: 0.44 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 4UY5 Resolution: 2.67→50.95 Å / SU ML: 0.34 / Cross valid method: THROUGHOUT / σ(F): 1.34 / Phase error: 27.39 / Stereochemistry target values: ML
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 47.56 Å2 / Biso mean: 29.4438 Å2 / Biso min: 21.03 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 2.67→50.95 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: X-RAY DIFFRACTION / Rfactor Rfree error: 0 / Total num. of bins used: 14
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Origin x: -22.245 Å / Origin y: -5.5358 Å / Origin z: 18.0802 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj



