+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-7609 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
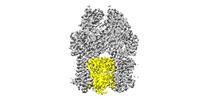
| Title | AcrBD407 mutant | |||||||||
 Map data Map data | primary map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | AcrB / Native cell membrane nanoparticles / SMA / lipid bilayer / TRANSPORT PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationalkane transmembrane transporter activity / alkane transport / enterobactin transport / enterobactin transmembrane transporter activity / xenobiotic detoxification by transmembrane export across the cell outer membrane / periplasmic side of plasma membrane / efflux pump complex / bile acid transmembrane transporter activity / xenobiotic transport / bile acid and bile salt transport ...alkane transmembrane transporter activity / alkane transport / enterobactin transport / enterobactin transmembrane transporter activity / xenobiotic detoxification by transmembrane export across the cell outer membrane / periplasmic side of plasma membrane / efflux pump complex / bile acid transmembrane transporter activity / xenobiotic transport / bile acid and bile salt transport / efflux transmembrane transporter activity / xenobiotic transmembrane transporter activity / fatty acid transport / response to toxic substance / response to xenobiotic stimulus / response to antibiotic / identical protein binding / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |   | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.0 Å | |||||||||
 Authors Authors | Qiu W / Fu Z | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2018 Journal: Proc Natl Acad Sci U S A / Year: 2018Title: Structure and activity of lipid bilayer within a membrane-protein transporter. Authors: Weihua Qiu / Ziao Fu / Guoyan G Xu / Robert A Grassucci / Yan Zhang / Joachim Frank / Wayne A Hendrickson / Youzhong Guo /  Abstract: Membrane proteins function in native cell membranes, but extraction into isolated particles is needed for many biochemical and structural analyses. Commonly used detergent-extraction methods destroy ...Membrane proteins function in native cell membranes, but extraction into isolated particles is needed for many biochemical and structural analyses. Commonly used detergent-extraction methods destroy naturally associated lipid bilayers. Here, we devised a detergent-free method for preparing cell-membrane nanoparticles to study the multidrug exporter AcrB, by cryo-EM at 3.2-Å resolution. We discovered a remarkably well-organized lipid-bilayer structure associated with transmembrane domains of the AcrB trimer. This bilayer patch comprises 24 lipid molecules; inner leaflet chains are packed in a hexagonal array, whereas the outer leaflet has highly irregular but ordered packing. Protein side chains interact with both leaflets and participate in the hexagonal pattern. We suggest that the lipid bilayer supports and harmonizes peristaltic motions through AcrB trimers. In AcrB D407A, a putative proton-relay mutant, lipid bilayer buttresses protein interactions lost in crystal structures after detergent-solubilization. Our detergent-free system preserves lipid-protein interactions for visualization and should be broadly applicable. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_7609.map.gz emd_7609.map.gz | 117.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-7609-v30.xml emd-7609-v30.xml emd-7609.xml emd-7609.xml | 15.2 KB 15.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_7609.png emd_7609.png | 164.8 KB | ||
| Filedesc metadata |  emd-7609.cif.gz emd-7609.cif.gz | 5.7 KB | ||
| Others |  emd_7609_additional_1.map.gz emd_7609_additional_1.map.gz emd_7609_additional_2.map.gz emd_7609_additional_2.map.gz | 117.1 MB 301.5 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-7609 http://ftp.pdbj.org/pub/emdb/structures/EMD-7609 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-7609 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-7609 | HTTPS FTP |
-Validation report
| Summary document |  emd_7609_validation.pdf.gz emd_7609_validation.pdf.gz | 662.4 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_7609_full_validation.pdf.gz emd_7609_full_validation.pdf.gz | 662 KB | Display | |
| Data in XML |  emd_7609_validation.xml.gz emd_7609_validation.xml.gz | 6.5 KB | Display | |
| Data in CIF |  emd_7609_validation.cif.gz emd_7609_validation.cif.gz | 7.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-7609 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-7609 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-7609 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-7609 | HTTPS FTP |
-Related structure data
| Related structure data |  6csxMC  7074C  6bajC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_7609.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_7609.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | primary map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.854 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
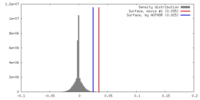
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: em-additional-volume P1
| File | emd_7609_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | em-additional-volume_P1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
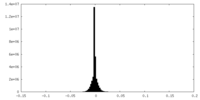
| Density Histograms |
-Additional map: lipid bilayer
| File | emd_7609_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | lipid bilayer | ||||||||||||
| Projections & Slices |
| ||||||||||||
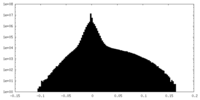
| Density Histograms |
- Sample components
Sample components
-Entire : Lipid bilayer in AcrB
| Entire | Name: Lipid bilayer in AcrB |
|---|---|
| Components |
|
-Supramolecule #1: Lipid bilayer in AcrB
| Supramolecule | Name: Lipid bilayer in AcrB / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Multidrug efflux pump subunit AcrB
| Macromolecule | Name: Multidrug efflux pump subunit AcrB / type: protein_or_peptide / ID: 1 / Details: AcrB, lipid bilayer / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 114.692289 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MPNFFIDRPI FAWVIAIIIM LAGGLAILKL PVAQYPTIAP PAVTISASYP GADAKTVQDT VTQVIEQNMN GIDNLMYMSS NSDSTGTVQ ITLTFESGTD ADIAQVQVQN KLQLAMPLLP QEVQQQGVSV EKSSSSFLMV VGVINTDGTM TQEDISDYVA A NMKDAISR ...String: MPNFFIDRPI FAWVIAIIIM LAGGLAILKL PVAQYPTIAP PAVTISASYP GADAKTVQDT VTQVIEQNMN GIDNLMYMSS NSDSTGTVQ ITLTFESGTD ADIAQVQVQN KLQLAMPLLP QEVQQQGVSV EKSSSSFLMV VGVINTDGTM TQEDISDYVA A NMKDAISR TSGVGDVQLF GSQYAMRIWM NPNELNKFQL TPVDVITAIK AQNAQVAAGQ LGGTPPVKGQ QLNASIIAQT RL TSTEEFG KILLKVNQDG SRVLLRDVAK IELGGENYDI IAEFNGQPAS GLGIKLATGA NALDTAAAIR AELAKMEPFF PSG LKIVYP YDTTPFVKIS IHEVVKTLVE AIILVFLVMY LFLQNFRATL IPTIAVPVVL LGTFAVLAAF GFSINTLTMF GMVL AIGLL VADAIVVVEN VERVMAEEGL PPKEATRKSM GQIQGALVGI AMVLSAVFVP MAFFGGSTGA IYRQFSITIV SAMAL SVLV ALILTPALCA TMLKPIAKGD HGEGKKGFFG WFNRMFEKST HHYTDSVGGI LRSTGRYLVL YLIIVVGMAY LFVRLP SSF LPDEDQGVFM TMVQLPAGAT QERTQKVLNE VTHYYLTKEK NNVESVFAVN GFGFAGRGQN TGIAFVSLKD WADRPGE EN KVEAITMRAT RAFSQIKDAM VFAFNLPAIV ELGTATGFDF ELIDQAGLGH EKLTQARNQL LAEAAKHPDM LTSVRPNG L EDTPQFKIDI DQEKAQALGV SINDINTTLG AAWGGSYVND FIDRGRVKKV YVMSEAKYRM LPDDIGDWYV RAADGQMVP FSAFSSSRWE YGSPRLERYN GLPSMEILGQ AAPGKSTGEA MELMEQLASK LPTGVGYDWT GMSYQERLSG NQAPSLYAIS LIVVFLCLA ALYESWSIPF SVMLVVPLGV IGALLAATFR GLTNDVYFQV GLLTTIGLSA KNAILIVEFA KDLMDKEGKG L IEATLDAV RMRLRPILMT SLAFILGVMP LVISTGAGSG AQNAVGTGVM GGMVTATVLA IFFVPVFFVV VRRRFSRKNE DI EHSHTVD HHLEHHHHHH UniProtKB: Multidrug efflux pump subunit AcrB |
-Macromolecule #2: PHOSPHATIDYLETHANOLAMINE
| Macromolecule | Name: PHOSPHATIDYLETHANOLAMINE / type: ligand / ID: 2 / Number of copies: 18 / Formula: PTY |
|---|---|
| Molecular weight | Theoretical: 734.039 Da |
| Chemical component information |  ChemComp-PTY: |
-Macromolecule #3: DODECANE
| Macromolecule | Name: DODECANE / type: ligand / ID: 3 / Number of copies: 1 / Formula: D12 |
|---|---|
| Molecular weight | Theoretical: 170.335 Da |
| Chemical component information |  ChemComp-D12: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.40 mg/mL |
|---|---|
| Buffer | pH: 7.8 |
| Grid | Material: GOLD |
| Vitrification | Cryogen name: ETHANE-PROPANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 45.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: PDB ENTRY |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.0 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 41190 |
| Initial angle assignment | Type: ANGULAR RECONSTITUTION |
| Final angle assignment | Type: ANGULAR RECONSTITUTION |
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)