[English] 日本語
 Yorodumi
Yorodumi- PDB-6rry: GOLGI ALPHA-MANNOSIDASE II in complex with (2S,3R)-2-(Hydroxymeth... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6rry | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | GOLGI ALPHA-MANNOSIDASE II in complex with (2S,3R)-2-(Hydroxymethyl)-1,2,3,6-tetrahydro-3-pyridinol | ||||||||||||
 Components Components | Alpha-mannosidase 2 | ||||||||||||
 Keywords Keywords | HYDROLASE / Mannosidase / Glycoside Hydrolase | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationmannosyl-oligosaccharide 1,3-1,6-alpha-mannosidase / mannosyl-oligosaccharide 1,3-1,6-alpha-mannosidase activity / rhodopsin biosynthetic process / encapsulation of foreign target / Reactions specific to the complex N-glycan synthesis pathway / mannosidase activity / alpha-mannosidase activity / N-glycan processing / mannose metabolic process / Golgi stack ...mannosyl-oligosaccharide 1,3-1,6-alpha-mannosidase / mannosyl-oligosaccharide 1,3-1,6-alpha-mannosidase activity / rhodopsin biosynthetic process / encapsulation of foreign target / Reactions specific to the complex N-glycan synthesis pathway / mannosidase activity / alpha-mannosidase activity / N-glycan processing / mannose metabolic process / Golgi stack / : / carbohydrate binding / Golgi membrane / endoplasmic reticulum / metal ion binding Similarity search - Function | ||||||||||||
| Biological species |  | ||||||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.86 Å MOLECULAR REPLACEMENT / Resolution: 1.86 Å | ||||||||||||
 Authors Authors | Armstrong, Z. / Lahav, D. / Johnson, R. / Kuo, C.L. / Beenakker, T.J.M. / de Boer, C. / Wong, C.S. / van Rijssel, E.R. / Debets, M. / Geurink, P.P. ...Armstrong, Z. / Lahav, D. / Johnson, R. / Kuo, C.L. / Beenakker, T.J.M. / de Boer, C. / Wong, C.S. / van Rijssel, E.R. / Debets, M. / Geurink, P.P. / Ovaa, H. / van der Stelt, M. / Codee, J.D.C. / Aerts, J.M.F.G. / Wu, L. / Overkleeft, H.S. / Davies, G.J. | ||||||||||||
| Funding support |  United Kingdom, United Kingdom,  Netherlands, 3items Netherlands, 3items
| ||||||||||||
 Citation Citation |  Journal: J.Am.Chem.Soc. / Year: 2020 Journal: J.Am.Chem.Soc. / Year: 2020Title: Manno- epi -cyclophellitols Enable Activity-Based Protein Profiling of Human alpha-Mannosidases and Discovery of New Golgi Mannosidase II Inhibitors. Authors: Armstrong, Z. / Kuo, C.L. / Lahav, D. / Liu, B. / Johnson, R. / Beenakker, T.J.M. / de Boer, C. / Wong, C.S. / van Rijssel, E.R. / Debets, M.F. / Florea, B.I. / Hissink, C. / Boot, R.G. / ...Authors: Armstrong, Z. / Kuo, C.L. / Lahav, D. / Liu, B. / Johnson, R. / Beenakker, T.J.M. / de Boer, C. / Wong, C.S. / van Rijssel, E.R. / Debets, M.F. / Florea, B.I. / Hissink, C. / Boot, R.G. / Geurink, P.P. / Ovaa, H. / van der Stelt, M. / van der Marel, G.M. / Codee, J.D.C. / Aerts, J.M.F.G. / Wu, L. / Overkleeft, H.S. / Davies, G.J. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6rry.cif.gz 6rry.cif.gz | 233.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6rry.ent.gz pdb6rry.ent.gz | 177.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6rry.json.gz 6rry.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/rr/6rry https://data.pdbj.org/pub/pdb/validation_reports/rr/6rry ftp://data.pdbj.org/pub/pdb/validation_reports/rr/6rry ftp://data.pdbj.org/pub/pdb/validation_reports/rr/6rry | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6rpcC  6rqzC  6rrhC  6rrjC  6rrnC  6rruC  6rrwC  6rrxC  6rs0C  3bubS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 118208.000 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Trichoplusia ni (cabbage looper) / Variant (production host): Hi Five Trichoplusia ni (cabbage looper) / Variant (production host): Hi FiveReferences: UniProt: Q24451, mannosyl-oligosaccharide 1,3-1,6-alpha-mannosidase |
|---|
-Non-polymers , 5 types, 496 molecules 
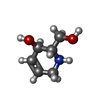







| #2: Chemical | | #3: Chemical | ChemComp-KGE / ( | #4: Chemical | ChemComp-SIN / | #5: Chemical | ChemComp-ZN / | #6: Water | ChemComp-HOH / | |
|---|
-Details
| Has protein modification | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.34 Å3/Da / Density % sol: 47.53 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, sitting drop Details: 0.1 M sodium succinate, pH 7.4 10 % PEG 3350 with microseeding |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Diamond Diamond  / Beamline: I04 / Wavelength: 0.95 Å / Beamline: I04 / Wavelength: 0.95 Å |
| Detector | Type: DECTRIS EIGER X 16M / Detector: PIXEL / Date: Mar 2, 2019 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.95 Å / Relative weight: 1 |
| Reflection | Resolution: 1.86→90.37 Å / Num. obs: 91011 / % possible obs: 100 % / Redundancy: 8.1 % / CC1/2: 0.996 / Net I/σ(I): 12.4 |
| Reflection shell | Resolution: 1.86→1.89 Å / Mean I/σ(I) obs: 1.3 / Num. unique obs: 4451 / CC1/2: 0.705 / % possible all: 100 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 3bub Resolution: 1.86→74.31 Å / Cor.coef. Fo:Fc: 0.966 / Cor.coef. Fo:Fc free: 0.946 / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R: 0.144 / ESU R Free: 0.14
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 102.11 Å2 / Biso mean: 34.862 Å2 / Biso min: 18.39 Å2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 1.86→74.31 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.86→1.908 Å / Total num. of bins used: 20
|
 Movie
Movie Controller
Controller














 PDBj
PDBj






