Entry Database : PDB / ID : 6bqjTitle CRYSTAL STRUCTURE OF HEPATIS C VIRUS PROTEASE (NS3) COMPLEXED WITH TRIPEPTIDIC ACYL SULFONAMIDE INHIBITOR (COMPOUND 16) NS3 protease Keywords / / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Method / / Resolution : 1.69 Å Authors Klei, H.E. / Sack, J.S. Journal : ACS Med Chem Lett / Year : 2018Title : Potent Inhibitors of Hepatitis C Virus NS3 Protease: Employment of a Difluoromethyl Group as a Hydrogen-Bond Donor.Authors: Zheng, B. / D'Andrea, S.V. / Sun, L.Q. / Wang, A.X. / Chen, Y. / Hrnciar, P. / Friborg, J. / Falk, P. / Hernandez, D. / Yu, F. / Sheaffer, A.K. / Knipe, J.O. / Mosure, K. / Rajamani, R. / ... Authors : Zheng, B. / D'Andrea, S.V. / Sun, L.Q. / Wang, A.X. / Chen, Y. / Hrnciar, P. / Friborg, J. / Falk, P. / Hernandez, D. / Yu, F. / Sheaffer, A.K. / Knipe, J.O. / Mosure, K. / Rajamani, R. / Good, A.C. / Kish, K. / Tredup, J. / Klei, H.E. / Paruchuri, M. / Ng, A. / Gao, Q. / Rampulla, R.A. / Mathur, A. / Meanwell, N.A. / McPhee, F. / Scola, P.M. History Deposition Nov 28, 2017 Deposition site / Processing site Revision 1.0 Mar 21, 2018 Provider / Type Revision 1.1 Sep 4, 2019 Group / Category / reflns_shellItem / _reflns_shell.pdbx_Rsym_valueRevision 1.2 Mar 13, 2024 Group / Data collection / Database referencesCategory chem_comp_atom / chem_comp_bond ... chem_comp_atom / chem_comp_bond / database_2 / pdbx_unobs_or_zero_occ_atoms Item / _database_2.pdbx_database_accession
Show all Show less
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information Hepatitis C virus
Hepatitis C virus X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON / Resolution: 1.69 Å
SYNCHROTRON / Resolution: 1.69 Å  Authors
Authors Citation
Citation Journal: ACS Med Chem Lett / Year: 2018
Journal: ACS Med Chem Lett / Year: 2018 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 6bqj.cif.gz
6bqj.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb6bqj.ent.gz
pdb6bqj.ent.gz PDB format
PDB format 6bqj.json.gz
6bqj.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/bq/6bqj
https://data.pdbj.org/pub/pdb/validation_reports/bq/6bqj ftp://data.pdbj.org/pub/pdb/validation_reports/bq/6bqj
ftp://data.pdbj.org/pub/pdb/validation_reports/bq/6bqj Links
Links Assembly
Assembly
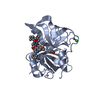


 Components
Components Hepatitis C virus / Production host:
Hepatitis C virus / Production host: 









 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  APS
APS  / Beamline: 32-ID / Wavelength: 1 Å
/ Beamline: 32-ID / Wavelength: 1 Å Processing
Processing Movie
Movie Controller
Controller






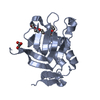






 PDBj
PDBj





