[English] 日本語
 Yorodumi
Yorodumi- PDB-5kbu: Cryo-EM structure of GluA2-2xSTZ complex at 7.8 Angstrom resolution -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5kbu | ||||||
|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of GluA2-2xSTZ complex at 7.8 Angstrom resolution | ||||||
 Components Components | Glutamate receptor 2,Voltage-dependent calcium channel gamma-2 subunit | ||||||
 Keywords Keywords | TRANSPORT PROTEIN / Cryo-EM | ||||||
| Function / homology |  Function and homology information Function and homology informationPresynaptic depolarization and calcium channel opening / LGI-ADAM interactions / Trafficking of AMPA receptors / eye blink reflex / positive regulation of protein localization to basolateral plasma membrane / cerebellar mossy fiber / postsynaptic neurotransmitter receptor diffusion trapping / regulation of AMPA receptor activity / membrane hyperpolarization / nervous system process ...Presynaptic depolarization and calcium channel opening / LGI-ADAM interactions / Trafficking of AMPA receptors / eye blink reflex / positive regulation of protein localization to basolateral plasma membrane / cerebellar mossy fiber / postsynaptic neurotransmitter receptor diffusion trapping / regulation of AMPA receptor activity / membrane hyperpolarization / nervous system process / protein targeting to membrane / voltage-gated calcium channel complex / spine synapse / dendritic spine neck / dendritic spine head / cellular response to amine stimulus / neurotransmitter receptor localization to postsynaptic specialization membrane / neuromuscular junction development / Activation of AMPA receptors / perisynaptic space / ligand-gated monoatomic cation channel activity / AMPA glutamate receptor activity / Trafficking of GluR2-containing AMPA receptors / transmission of nerve impulse / response to lithium ion / kainate selective glutamate receptor activity / cellular response to glycine / AMPA glutamate receptor complex / extracellularly glutamate-gated ion channel activity / immunoglobulin binding / asymmetric synapse / ionotropic glutamate receptor complex / conditioned place preference / regulation of receptor recycling / membrane depolarization / glutamate receptor binding / Unblocking of NMDA receptors, glutamate binding and activation / positive regulation of synaptic transmission / regulation of postsynaptic membrane neurotransmitter receptor levels / regulation of synaptic transmission, glutamatergic / voltage-gated calcium channel activity / response to fungicide / glutamate-gated receptor activity / regulation of long-term synaptic depression / cytoskeletal protein binding / extracellular ligand-gated monoatomic ion channel activity / cellular response to brain-derived neurotrophic factor stimulus / glutamate-gated calcium ion channel activity / presynaptic active zone membrane / somatodendritic compartment / ionotropic glutamate receptor binding / dendrite membrane / ligand-gated monoatomic ion channel activity involved in regulation of presynaptic membrane potential / ionotropic glutamate receptor signaling pathway / dendrite cytoplasm / synaptic membrane / hippocampal mossy fiber to CA3 synapse / dendritic shaft / SNARE binding / regulation of membrane potential / PDZ domain binding / transmitter-gated monoatomic ion channel activity involved in regulation of postsynaptic membrane potential / synaptic transmission, glutamatergic / protein tetramerization / establishment of protein localization / response to calcium ion / postsynaptic density membrane / cerebral cortex development / modulation of chemical synaptic transmission / receptor internalization / Schaffer collateral - CA1 synapse / terminal bouton / synaptic vesicle / synaptic vesicle membrane / signaling receptor activity / presynapse / amyloid-beta binding / growth cone / presynaptic membrane / scaffold protein binding / perikaryon / dendritic spine / chemical synaptic transmission / postsynaptic membrane / neuron projection / postsynaptic density / axon / external side of plasma membrane / neuronal cell body / synapse / dendrite / protein kinase binding / protein-containing complex binding / glutamatergic synapse / cell surface / endoplasmic reticulum / protein-containing complex / identical protein binding / membrane / plasma membrane Similarity search - Function | ||||||
| Biological species |   | ||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 7.8 Å | ||||||
 Authors Authors | Twomey, E.C. / Yelshanskaya, M.V. / Grassucci, R.A. / Frank, J. / Sobolevsky, A.I. | ||||||
| Funding support |  United States, 1items United States, 1items
| ||||||
 Citation Citation |  Journal: Science / Year: 2016 Journal: Science / Year: 2016Title: Elucidation of AMPA receptor-stargazin complexes by cryo-electron microscopy. Authors: Edward C Twomey / Maria V Yelshanskaya / Robert A Grassucci / Joachim Frank / Alexander I Sobolevsky /  Abstract: AMPA-subtype ionotropic glutamate receptors (AMPARs) mediate fast excitatory neurotransmission and contribute to high cognitive processes such as learning and memory. In the brain, AMPAR trafficking, ...AMPA-subtype ionotropic glutamate receptors (AMPARs) mediate fast excitatory neurotransmission and contribute to high cognitive processes such as learning and memory. In the brain, AMPAR trafficking, gating, and pharmacology is tightly controlled by transmembrane AMPAR regulatory proteins (TARPs). Here, we used cryo-electron microscopy to elucidate the structural basis of AMPAR regulation by one of these auxiliary proteins, TARP γ2, or stargazin (STZ). Our structures illuminate the variable interaction stoichiometry of the AMPAR-TARP complex, with one or two TARP molecules binding one tetrameric AMPAR. Analysis of the AMPAR-STZ binding interfaces suggests that electrostatic interactions between the extracellular domains of AMPAR and STZ play an important role in modulating AMPAR function through contact surfaces that are conserved across AMPARs and TARPs. We propose a model explaining how TARPs stabilize the activated state of AMPARs and how the interactions between AMPARs and their auxiliary proteins control fast excitatory synaptic transmission. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5kbu.cif.gz 5kbu.cif.gz | 665.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5kbu.ent.gz pdb5kbu.ent.gz | 519 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5kbu.json.gz 5kbu.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  5kbu_validation.pdf.gz 5kbu_validation.pdf.gz | 1.1 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  5kbu_full_validation.pdf.gz 5kbu_full_validation.pdf.gz | 1.3 MB | Display | |
| Data in XML |  5kbu_validation.xml.gz 5kbu_validation.xml.gz | 124.3 KB | Display | |
| Data in CIF |  5kbu_validation.cif.gz 5kbu_validation.cif.gz | 174.1 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/kb/5kbu https://data.pdbj.org/pub/pdb/validation_reports/kb/5kbu ftp://data.pdbj.org/pub/pdb/validation_reports/kb/5kbu ftp://data.pdbj.org/pub/pdb/validation_reports/kb/5kbu | HTTPS FTP |
-Related structure data
| Related structure data |  8231MC  8229C  8230C  8232C  5kbsC  5kbtC  5kbvC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
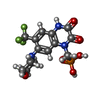
| #1: Protein | Mass: 115515.984 Da / Num. of mol.: 4 / Mutation: N241E, V382L, G384E, N385D, V758L Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Gene: Gria2, Glur2, Cacng2, Stg / Production host:  Homo sapiens (human) / References: UniProt: P19491, UniProt: O88602 Homo sapiens (human) / References: UniProt: P19491, UniProt: O88602#2: Chemical | ChemComp-ZK1 / {[ #3: Sugar | ChemComp-NAG / Compound details | The protein was expressed as a tandem fusion construct (GluA2 fused to stargazin, also named TARP- ...The protein was expressed as a tandem fusion construct (GluA2 fused to stargazin, also named TARP-y2 or cagcn2). However, this second protein which is covalently linked or fused to the C-terminus of GluA2 is not observed in chains B and D in this structure. | Has protein modification | Y | |
|---|
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Protein / Type: COMPLEX / Entity ID: all / Source: RECOMBINANT |
|---|---|
| Source (natural) | Organism:  |
| Source (recombinant) | Organism:  Homo sapiens (human) / Cell: HEK293 / Plasmid: Bacmam Homo sapiens (human) / Cell: HEK293 / Plasmid: Bacmam |
| Buffer solution | pH: 8 |
| Specimen | Conc.: 2 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Specimen support | Details: Grid coated with gold prior to use. / Grid material: GOLD / Grid mesh size: 200 divisions/in. / Grid type: C-flat Au 1.2/1.3 |
| Vitrification | Instrument: FEI VITROBOT MARK IV / Cryogen name: ETHANE / Humidity: 100 % / Chamber temperature: 295 K / Details: 3 blot force, 8.0 s blot time |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: SPOT SCAN FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: SPOT SCAN |
| Electron lens | Mode: BRIGHT FIELD |
| Specimen holder | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Image recording | Average exposure time: 8 sec. / Electron dose: 80 e/Å2 / Detector mode: SUPER-RESOLUTION / Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Num. of grids imaged: 1 Details: 40 frames were collected across 8 seconds per image. |
| Image scans | Movie frames/image: 40 / Used frames/image: 1-40 |
- Processing
Processing
| EM software |
| ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||
| Symmetry | Point symmetry: C2 (2 fold cyclic) | ||||||||||||||||||||
| 3D reconstruction | Resolution: 7.8 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 10293 / Symmetry type: POINT |
 Movie
Movie Controller
Controller











 PDBj
PDBj