[English] 日本語
 Yorodumi
Yorodumi- EMDB-4779: Heterodimeric ABC exporter TmrAB under turnover conditions in asy... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-4779 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Heterodimeric ABC exporter TmrAB under turnover conditions in asymmetric unlocked return conformation | ||||||||||||
 Map data Map data | TmrAB in unlocked return conformation under turnover conditions | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | ATP-binding cassette transporter / membrane protein / heterodimer / exporter / TRANSPORT PROTEIN | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationABC-type oligopeptide transporter activity / ATP hydrolysis activity / ATP binding / plasma membrane Similarity search - Function | ||||||||||||
| Biological species |   Thermus thermophilus (bacteria) / Thermus thermophilus (bacteria) /  | ||||||||||||
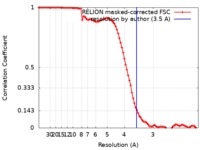
| Method | single particle reconstruction / cryo EM / Resolution: 3.5 Å | ||||||||||||
 Authors Authors | Januliene D / Hofmann S / Medhdipour AR / Thomas C / Hummer G / Tampe R / Moeller A | ||||||||||||
| Funding support |  Germany, 3 items Germany, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nature / Year: 2019 Journal: Nature / Year: 2019Title: Conformation space of a heterodimeric ABC exporter under turnover conditions. Authors: Susanne Hofmann / Dovile Januliene / Ahmad R Mehdipour / Christoph Thomas / Erich Stefan / Stefan Brüchert / Benedikt T Kuhn / Eric R Geertsma / Gerhard Hummer / Robert Tampé / Arne Moeller /  Abstract: Cryo-electron microscopy (cryo-EM) has the capacity to capture molecular machines in action. ATP-binding cassette (ABC) exporters are highly dynamic membrane proteins that extrude a wide range of ...Cryo-electron microscopy (cryo-EM) has the capacity to capture molecular machines in action. ATP-binding cassette (ABC) exporters are highly dynamic membrane proteins that extrude a wide range of substances from the cytosol and thereby contribute to essential cellular processes, adaptive immunity and multidrug resistance. Despite their importance, the coupling of nucleotide binding, hydrolysis and release to the conformational dynamics of these proteins remains poorly resolved, especially for heterodimeric and/or asymmetric ABC exporters that are abundant in humans. Here we present eight high-resolution cryo-EM structures that delineate the full functional cycle of an asymmetric ABC exporter in a lipid environment. Cryo-EM analysis under active turnover conditions reveals distinct inward-facing (IF) conformations-one of them with a bound peptide substrate-and previously undescribed asymmetric post-hydrolysis states with dimerized nucleotide-binding domains and a closed extracellular gate. By decreasing the rate of ATP hydrolysis, we could capture an outward-facing (OF) open conformation-an otherwise transient state vulnerable to substrate re-entry. The ATP-bound pre-hydrolysis and vanadate-trapped states are conformationally equivalent; both comprise co-existing OF conformations with open and closed extracellular gates. By contrast, the post-hydrolysis states from the turnover experiment exhibit asymmetric ATP and ADP occlusion after phosphate release from the canonical site and display a progressive separation of the nucleotide-binding domains and unlocking of the intracellular gate. Our findings reveal that phosphate release, not ATP hydrolysis, triggers the return of the exporter to the IF conformation. By mapping the conformational landscape during active turnover, aided by mutational and chemical modulation of kinetic rates to trap the key intermediates, we resolved fundamental steps of the substrate translocation cycle of asymmetric ABC transporters. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_4779.map.gz emd_4779.map.gz | 4.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-4779-v30.xml emd-4779-v30.xml emd-4779.xml emd-4779.xml | 27.3 KB 27.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_4779_fsc.xml emd_4779_fsc.xml | 9.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_4779.png emd_4779.png | 41.6 KB | ||
| Masks |  emd_4779_msk_1.map emd_4779_msk_1.map | 64 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-4779.cif.gz emd-4779.cif.gz | 7.9 KB | ||
| Others |  emd_4779_half_map_1.map.gz emd_4779_half_map_1.map.gz emd_4779_half_map_2.map.gz emd_4779_half_map_2.map.gz | 59.4 MB 59.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-4779 http://ftp.pdbj.org/pub/emdb/structures/EMD-4779 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4779 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4779 | HTTPS FTP |
-Related structure data
| Related structure data |  6ralMC  4773C  4774C  4775C  4776C  4777C  4778C  4780C  4781C  6rafC  6ragC  6rahC  6raiC  6rajC  6rakC  6ramC  6ranC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_4779.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_4779.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | TmrAB in unlocked return conformation under turnover conditions | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.077 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_4779_msk_1.map emd_4779_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map 1
| File | emd_4779_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map 2
| File | emd_4779_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : TmrAB in asymmetric unlocked return conformation under turnover c...
+Supramolecule #1: TmrAB in asymmetric unlocked return conformation under turnover c...
+Supramolecule #2: Multidrug resistance ABC transporter ATP-binding and permease protein
+Supramolecule #3: Multidrug resistance ABC transporter ATP-binding and permease protein
+Supramolecule #4: Nanobody Nb9F10
+Macromolecule #1: Multidrug resistance ABC transporter ATP-binding and permease protein
+Macromolecule #2: Multidrug resistance ABC transporter ATP-binding and permease protein
+Macromolecule #3: Nanobody Nb9F10
+Macromolecule #4: ADENOSINE-5'-DIPHOSPHATE
+Macromolecule #5: MAGNESIUM ION
+Macromolecule #6: ADENOSINE-5'-TRIPHOSPHATE
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Grid | Model: Quantifoil, UltrAuFoil / Material: GOLD / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Detector mode: COUNTING / Average exposure time: 8.0 sec. / Average electron dose: 62.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)