+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | HCN1 complex with propofol | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | inhibitor / complex / plasma membrane / cyclic nucleotide / TRANSPORT PROTEIN-INHIBITOR complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationintracellular cAMP-activated cation channel activity involved in regulation of presynaptic membrane potential / HCN channels / general adaptation syndrome, behavioral process / HCN channel complex / retinal cone cell development / regulation of membrane depolarization / intracellularly cAMP-activated cation channel activity / apical protein localization / voltage-gated monoatomic cation channel activity / voltage-gated sodium channel activity ...intracellular cAMP-activated cation channel activity involved in regulation of presynaptic membrane potential / HCN channels / general adaptation syndrome, behavioral process / HCN channel complex / retinal cone cell development / regulation of membrane depolarization / intracellularly cAMP-activated cation channel activity / apical protein localization / voltage-gated monoatomic cation channel activity / voltage-gated sodium channel activity / voltage-gated potassium channel activity / potassium channel activity / sodium ion transmembrane transport / neuronal action potential / cAMP binding / cellular response to cAMP / presynaptic active zone membrane / potassium ion transmembrane transport / regulation of membrane potential / postsynaptic membrane / protein homotetramerization / axon / glutamatergic synapse / dendrite / identical protein binding / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
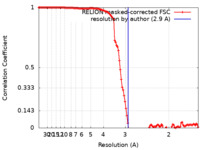
| Method | single particle reconstruction / cryo EM / Resolution: 2.9 Å | |||||||||
 Authors Authors | Kim ED / Nimigean CM | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2024 Journal: Nature / Year: 2024Title: Propofol rescues voltage-dependent gating of HCN1 channel epilepsy mutants Authors: Kim ED / Wu X / Lee S / Tibbs GR / Cunningham KP / Perez ME / Goldstein PA / Accardi A / Larsson HP / Nimigean CM | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_42116.map.gz emd_42116.map.gz | 138 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-42116-v30.xml emd-42116-v30.xml emd-42116.xml emd-42116.xml | 16.6 KB 16.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_42116_fsc.xml emd_42116_fsc.xml | 12.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_42116.png emd_42116.png | 77.4 KB | ||
| Filedesc metadata |  emd-42116.cif.gz emd-42116.cif.gz | 5.9 KB | ||
| Others |  emd_42116_additional_2.map.gz emd_42116_additional_2.map.gz emd_42116_half_map_1.map.gz emd_42116_half_map_1.map.gz emd_42116_half_map_2.map.gz emd_42116_half_map_2.map.gz | 134 MB 112.7 MB 112.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-42116 http://ftp.pdbj.org/pub/emdb/structures/EMD-42116 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42116 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42116 | HTTPS FTP |
-Validation report
| Summary document |  emd_42116_validation.pdf.gz emd_42116_validation.pdf.gz | 757.2 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_42116_full_validation.pdf.gz emd_42116_full_validation.pdf.gz | 756.7 KB | Display | |
| Data in XML |  emd_42116_validation.xml.gz emd_42116_validation.xml.gz | 19.3 KB | Display | |
| Data in CIF |  emd_42116_validation.cif.gz emd_42116_validation.cif.gz | 25.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42116 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42116 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42116 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42116 | HTTPS FTP |
-Related structure data
| Related structure data |  8uc7MC  8uc8C  9bc6C  9bc7C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_42116.map.gz / Format: CCP4 / Size: 149.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_42116.map.gz / Format: CCP4 / Size: 149.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.815 Å | ||||||||||||||||||||||||||||||||||||
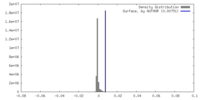
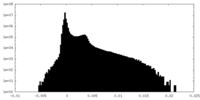
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: #1
| File | emd_42116_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
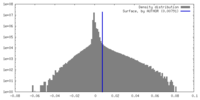
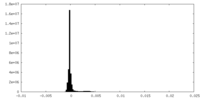
| Density Histograms |
-Half map: #2
| File | emd_42116_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
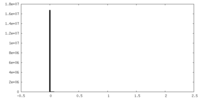
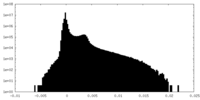
| Density Histograms |
-Half map: #1
| File | emd_42116_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
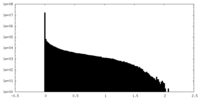
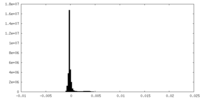
| Density Histograms |
- Sample components
Sample components
-Entire : HCN1 with propofol
| Entire | Name: HCN1 with propofol |
|---|---|
| Components |
|
-Supramolecule #1: HCN1 with propofol
| Supramolecule | Name: HCN1 with propofol / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Potassium/sodium hyperpolarization-activated cyclic nucleotide-ga...
| Macromolecule | Name: Potassium/sodium hyperpolarization-activated cyclic nucleotide-gated channel 1 type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 74.643734 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MEGGGKPNSS SNSRDDGNSV FPAKASATGA GPAAAEKRLG TPPGGGGAGA KEHGNSVCFK VDGGGGGGGG GGGGEEPAGG FEDAEGPRR QYGFMQRQFT SMLQPGVNKF SLRMFGSQKA VEKEQERVKT AGFWIIHPYS DFRFYWDLIM LIMMVGNLVI I PVGITFFT ...String: MEGGGKPNSS SNSRDDGNSV FPAKASATGA GPAAAEKRLG TPPGGGGAGA KEHGNSVCFK VDGGGGGGGG GGGGEEPAGG FEDAEGPRR QYGFMQRQFT SMLQPGVNKF SLRMFGSQKA VEKEQERVKT AGFWIIHPYS DFRFYWDLIM LIMMVGNLVI I PVGITFFT EQTTTPWIIF NVASDTVFLL DLIMNFRTGT VNEDSSEIIL DPKVIKMNYL KSWFVVDFIS SIPVDYIFLI VE KGMDSEV YKTARALRIV RFTKILSLLR LLRLSRLIRY IHQWEEIFHM TYDLASAVVR IFNLIGMMLL LCHWDGCLQF LVP LLQDFP PDCWVSLNEM VNDSWGKQYS YALFKAMSHM LCIGYGAQAP VSMSDLWITM LSMIVGATCY AMFVGHATAL IQSL DSSRR QYQEKYKQVE QYMSFHKLPA DMRQKIHDYY EHRYQGKIFD EENILNELND PLREEIVNFN CRKLVATMPL FANAD PNFV TAMLSKLRFE VFQPGDYIIR EGAVGKKMYF IQHGVAGVIT KSSKEMKLTD GSYFGEICLL TKGRRTASVR ADTYCR LYS LSVDNFNEVL EEYPMMRRAF ETVAIDRLDR IGKKNSILLQ KFQKDLNTGV FNNQENEILK QIVKHDREMV QAALPRE SS SVLNTDPDAE KPRFASNL UniProtKB: Potassium/sodium hyperpolarization-activated cyclic nucleotide-gated channel 1, Potassium/sodium hyperpolarization-activated cyclic nucleotide-gated channel 1 |
-Macromolecule #2: 2,6-BIS(1-METHYLETHYL)PHENOL
| Macromolecule | Name: 2,6-BIS(1-METHYLETHYL)PHENOL / type: ligand / ID: 2 / Number of copies: 4 / Formula: PFL |
|---|---|
| Molecular weight | Theoretical: 178.271 Da |
| Chemical component information | 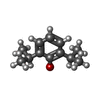 ChemComp-PFL: |
-Macromolecule #3: 1,2-DIOLEOYL-SN-GLYCERO-3-PHOSPHOCHOLINE
| Macromolecule | Name: 1,2-DIOLEOYL-SN-GLYCERO-3-PHOSPHOCHOLINE / type: ligand / ID: 3 / Number of copies: 12 / Formula: PCW |
|---|---|
| Molecular weight | Theoretical: 787.121 Da |
| Chemical component information |  ChemComp-PCW: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 52.53 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)