+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 |  | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | Major polymorph in alpha-synuclein fibril seeded by cerebrospinal fluid from a mid-to-late stage (mid-PD-1) Parkinson's disease patient | |||||||||||||||||||||||||||
 マップデータ マップデータ | ||||||||||||||||||||||||||||
 試料 試料 |
| |||||||||||||||||||||||||||
 キーワード キーワード | amyloid fibril / PROTEIN FIBRIL | |||||||||||||||||||||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報regulation of phospholipase activity / negative regulation of monooxygenase activity / negative regulation of mitochondrial electron transport, NADH to ubiquinone / positive regulation of glutathione peroxidase activity / neutral lipid metabolic process / regulation of acyl-CoA biosynthetic process / negative regulation of dopamine uptake involved in synaptic transmission / negative regulation of norepinephrine uptake / positive regulation of SNARE complex assembly / positive regulation of hydrogen peroxide catabolic process ...regulation of phospholipase activity / negative regulation of monooxygenase activity / negative regulation of mitochondrial electron transport, NADH to ubiquinone / positive regulation of glutathione peroxidase activity / neutral lipid metabolic process / regulation of acyl-CoA biosynthetic process / negative regulation of dopamine uptake involved in synaptic transmission / negative regulation of norepinephrine uptake / positive regulation of SNARE complex assembly / positive regulation of hydrogen peroxide catabolic process / supramolecular fiber / negative regulation of transporter activity / mitochondrial membrane organization / negative regulation of chaperone-mediated autophagy / regulation of reactive oxygen species biosynthetic process / regulation of synaptic vesicle recycling / negative regulation of platelet-derived growth factor receptor signaling pathway / positive regulation of protein localization to cell periphery / negative regulation of exocytosis / regulation of glutamate secretion / response to iron(II) ion / regulation of norepinephrine uptake / SNARE complex assembly / positive regulation of neurotransmitter secretion / dopamine biosynthetic process / regulation of locomotion / positive regulation of inositol phosphate biosynthetic process / synaptic vesicle priming / regulation of macrophage activation / negative regulation of microtubule polymerization / synaptic vesicle transport / dynein complex binding / dopamine uptake involved in synaptic transmission / positive regulation of receptor recycling / regulation of dopamine secretion / protein kinase inhibitor activity / negative regulation of thrombin-activated receptor signaling pathway / cuprous ion binding / response to magnesium ion / response to type II interferon / positive regulation of exocytosis / synaptic vesicle exocytosis / positive regulation of endocytosis / kinesin binding / cysteine-type endopeptidase inhibitor activity involved in apoptotic process / mitochondrial ATP synthesis coupled electron transport / synaptic vesicle endocytosis / regulation of presynapse assembly / negative regulation of serotonin uptake / alpha-tubulin binding / phospholipid metabolic process / supramolecular fiber organization / axon terminus / inclusion body / cellular response to copper ion / cellular response to epinephrine stimulus / Hsp70 protein binding / response to interleukin-1 / : / adult locomotory behavior / positive regulation of release of sequestered calcium ion into cytosol / SNARE binding / excitatory postsynaptic potential / fatty acid metabolic process / long-term synaptic potentiation / phosphoprotein binding / protein tetramerization / regulation of transmembrane transporter activity / protein destabilization / negative regulation of protein kinase activity / microglial cell activation / regulation of long-term neuronal synaptic plasticity / synapse organization / ferrous iron binding / positive regulation of protein serine/threonine kinase activity / tau protein binding / PKR-mediated signaling / receptor internalization / : / phospholipid binding / synaptic vesicle membrane / positive regulation of inflammatory response / actin cytoskeleton / positive regulation of peptidyl-serine phosphorylation / actin binding / cell cortex / cellular response to oxidative stress / histone binding / growth cone / chemical synaptic transmission / neuron apoptotic process / negative regulation of neuron apoptotic process / postsynapse / response to lipopolysaccharide / amyloid fibril formation / molecular adaptor activity / lysosome / transcription cis-regulatory region binding / oxidoreductase activity / positive regulation of apoptotic process 類似検索 - 分子機能 | |||||||||||||||||||||||||||
| 生物種 |  Homo sapiens (ヒト) Homo sapiens (ヒト) | |||||||||||||||||||||||||||
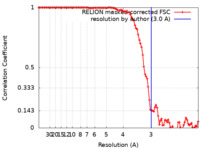
| 手法 | らせん対称体再構成法 / クライオ電子顕微鏡法 / 解像度: 3.0 Å | |||||||||||||||||||||||||||
 データ登録者 データ登録者 | Fan Y / Sun YP / Wang J / Liu C | |||||||||||||||||||||||||||
| 資金援助 |  中国, 8件 中国, 8件
| |||||||||||||||||||||||||||
 引用 引用 |  ジャーナル: Structure / 年: 2023 ジャーナル: Structure / 年: 2023タイトル: Conformational change of α-synuclein fibrils in cerebrospinal fluid from different clinical phases of Parkinson's disease. 著者: Yun Fan / Yunpeng Sun / Wenbo Yu / Youqi Tao / Wencheng Xia / Yiqi Liu / Qinyue Zhao / Yilin Tang / Yimin Sun / Fengtao Liu / Qin Cao / Jianjun Wu / Cong Liu / Jian Wang / Dan Li /  要旨: α-Synuclein (α-syn) has been shown to form various conformational fibrils associated with different synucleinopathies. But whether the conformation of α-syn fibrils changes during disease ...α-Synuclein (α-syn) has been shown to form various conformational fibrils associated with different synucleinopathies. But whether the conformation of α-syn fibrils changes during disease progression is unclear. Here, we amplified α-syn aggregates from the cerebrospinal fluid (CSF) of patients with Parkinson's disease (PD) staged in preclinical PD (pre-PD), middle- to late-stage PD (mid-PD), and late-stage PD (late-PD). Our results show that α-syn fibrils derived from the late-PD patient are most potent in inducing endogenous α-syn aggregation in primary neurons, followed by the mid-PD and pre-PD fibrils. By using cryo-electron microscopy, we further determined the high-resolution structures of the CSF-amplified fibrils. The structures exhibit remarkable differences in a minor but significant population of conformational species in different staged samples. Our work demonstrates structural and pathological differences between α-syn fibrils derived from PD patients at a spectrum of clinical stages, which suggests potential conformational transition of α-syn fibrils during the progression of PD. | |||||||||||||||||||||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| 添付画像 |
|---|
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_33333.map.gz emd_33333.map.gz | 15.3 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-33333-v30.xml emd-33333-v30.xml emd-33333.xml emd-33333.xml | 15.5 KB 15.5 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| FSC (解像度算出) |  emd_33333_fsc.xml emd_33333_fsc.xml | 10.2 KB | 表示 |  FSCデータファイル FSCデータファイル |
| 画像 |  emd_33333.png emd_33333.png | 40 KB | ||
| Filedesc metadata |  emd-33333.cif.gz emd-33333.cif.gz | 5.2 KB | ||
| その他 |  emd_33333_half_map_1.map.gz emd_33333_half_map_1.map.gz emd_33333_half_map_2.map.gz emd_33333_half_map_2.map.gz | 70.6 MB 70.6 MB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-33333 http://ftp.pdbj.org/pub/emdb/structures/EMD-33333 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33333 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33333 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_33333_validation.pdf.gz emd_33333_validation.pdf.gz | 718.6 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_33333_full_validation.pdf.gz emd_33333_full_validation.pdf.gz | 718.2 KB | 表示 | |
| XML形式データ |  emd_33333_validation.xml.gz emd_33333_validation.xml.gz | 17.5 KB | 表示 | |
| CIF形式データ |  emd_33333_validation.cif.gz emd_33333_validation.cif.gz | 23 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33333 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33333 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33333 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33333 | HTTPS FTP |
-関連構造データ
| 関連構造データ |  7xo1MC  7v47C  7v48C  7v49C  7xo0C  7xo2C  7xo3C  8h03C  8h04C  8h05C M: このマップから作成された原子モデル C: 同じ文献を引用 ( |
|---|---|
| 類似構造データ | 類似検索 - 機能・相同性  F&H 検索 F&H 検索 |
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_33333.map.gz / 形式: CCP4 / 大きさ: 91.1 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_33333.map.gz / 形式: CCP4 / 大きさ: 91.1 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 1.06 Å | ||||||||||||||||||||||||||||||||||||
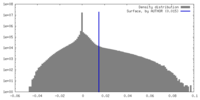
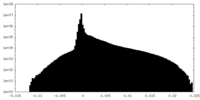
| 密度 |
| ||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
|
-添付データ
-ハーフマップ: #2
| ファイル | emd_33333_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
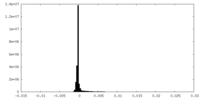
| 投影像・断面図 |
| ||||||||||||
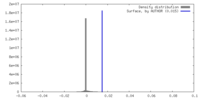
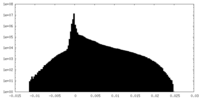
| 密度ヒストグラム |
-ハーフマップ: #1
| ファイル | emd_33333_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 投影像・断面図 |
| ||||||||||||
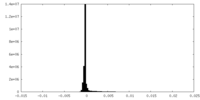
| 密度ヒストグラム |
- 試料の構成要素
試料の構成要素
-全体 : Major polymorph in alpha-synuclein fibril seeded by cerebrospinal...
| 全体 | 名称: Major polymorph in alpha-synuclein fibril seeded by cerebrospinal fluid from a mid-to-late stage (mid-PD-1) Parkinson's disease patient |
|---|---|
| 要素 |
|
-超分子 #1: Major polymorph in alpha-synuclein fibril seeded by cerebrospinal...
| 超分子 | 名称: Major polymorph in alpha-synuclein fibril seeded by cerebrospinal fluid from a mid-to-late stage (mid-PD-1) Parkinson's disease patient タイプ: organelle_or_cellular_component / ID: 1 / 親要素: 0 / 含まれる分子: all |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
-分子 #1: Alpha-synuclein
| 分子 | 名称: Alpha-synuclein / タイプ: protein_or_peptide / ID: 1 / コピー数: 6 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 14.476108 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: MDVFMKGLSK AKEGVVAAAE KTKQGVAEAA GKTKEGVLYV GSKTKEGVVH GVATVAEKTK EQVTNVGGAV VTGVTAVAQK TVEGAGSIA AATGFVKKDQ LGKNEEGAPQ EGILEDMPVD PDNEAYEMPS EEGYQDYEPE A UniProtKB: Alpha-synuclein |
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | らせん対称体再構成法 |
| 試料の集合状態 | filament |
- 試料調製
試料調製
| 緩衝液 | pH: 6.5 |
|---|---|
| 凍結 | 凍結剤: ETHANE |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TITAN KRIOS |
|---|---|
| 撮影 | フィルム・検出器のモデル: GATAN K3 (6k x 4k) / 平均電子線量: 55.0 e/Å2 |
| 電子線 | 加速電圧: 300 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD / 最大 デフォーカス(公称値): 2.0 µm / 最小 デフォーカス(公称値): 1.0 µm |
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
 ムービー
ムービー コントローラー
コントローラー










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)