[English] 日本語
 Yorodumi
Yorodumi- EMDB-32612: Structure of apo-ferritin with Hybrid electron counting at 120 kV -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of apo-ferritin with Hybrid electron counting at 120 kV | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Coxsackievirus A10 / VIRUS | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
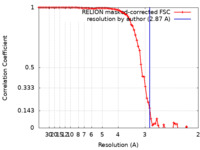
| Method | single particle reconstruction / cryo EM / Resolution: 2.87 Å | |||||||||
 Authors Authors | Zhu DJ / Zhang XZ | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Commun Biol / Year: 2022 Journal: Commun Biol / Year: 2022Title: An electron counting algorithm improves imaging of proteins with low-acceleration-voltage cryo-electron microscope. Authors: Dongjie Zhu / Huigang Shi / Chunling Wu / Xinzheng Zhang /  Abstract: Relative to the 300-kV accelerating field, electrons accelerated under lower voltages are potentially scattered more strongly. Lowering the accelerate voltage has been suggested to enhance the signal- ...Relative to the 300-kV accelerating field, electrons accelerated under lower voltages are potentially scattered more strongly. Lowering the accelerate voltage has been suggested to enhance the signal-to-noise ratio (SNR) of cryo-electron microscopy (cryo-EM) images of small-molecular-weight proteins (<100 kD). However, the detection efficient of current Direct Detection Devices (DDDs) and temporal coherence of cryo-EM decrease at lower voltage, leading to loss of SNR. Here, we present an electron counting algorithm to improve the detection of low-energy electrons. The counting algorithm increased the SNR of 120-kV and 200-kV cryo-EM image from a Falcon III camera by 8%, 20% at half the Nyquist frequency and 21%, 80% at Nyquist frequency, respectively, resulting in a considerable improvement in resolution of 3D reconstructions. Our results indicate that with further improved temporal coherence and a dedicated designed camera, a 120-kV cryo-electron microscope has potential to match the 300-kV microscope at imaging small proteins. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_32612.map.gz emd_32612.map.gz | 40.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-32612-v30.xml emd-32612-v30.xml emd-32612.xml emd-32612.xml | 12.3 KB 12.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_32612_fsc.xml emd_32612_fsc.xml | 8 KB | Display |  FSC data file FSC data file |
| Images |  emd_32612.png emd_32612.png | 150.7 KB | ||
| Masks |  emd_32612_msk_1.map emd_32612_msk_1.map | 42.9 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-32612.cif.gz emd-32612.cif.gz | 3.8 KB | ||
| Others |  emd_32612_half_map_1.map.gz emd_32612_half_map_1.map.gz emd_32612_half_map_2.map.gz emd_32612_half_map_2.map.gz | 29.6 MB 29.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-32612 http://ftp.pdbj.org/pub/emdb/structures/EMD-32612 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32612 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32612 | HTTPS FTP |
-Validation report
| Summary document |  emd_32612_validation.pdf.gz emd_32612_validation.pdf.gz | 824 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_32612_full_validation.pdf.gz emd_32612_full_validation.pdf.gz | 823.6 KB | Display | |
| Data in XML |  emd_32612_validation.xml.gz emd_32612_validation.xml.gz | 12.9 KB | Display | |
| Data in CIF |  emd_32612_validation.cif.gz emd_32612_validation.cif.gz | 18.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32612 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32612 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32612 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32612 | HTTPS FTP |
-Related structure data
| Related structure data | C: citing same article ( |
|---|
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_32612.map.gz / Format: CCP4 / Size: 42.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_32612.map.gz / Format: CCP4 / Size: 42.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1 Å | ||||||||||||||||||||||||||||||||||||
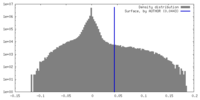
| Density |
| ||||||||||||||||||||||||||||||||||||
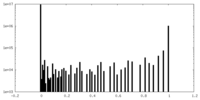
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_32612_msk_1.map emd_32612_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
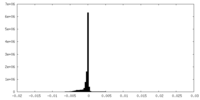
| Density Histograms |
-Half map: #2
| File | emd_32612_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
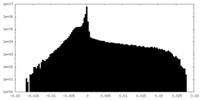
| Density Histograms |
-Half map: #1
| File | emd_32612_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : apo-ferritin
| Entire | Name: apo-ferritin |
|---|---|
| Components |
|
-Supramolecule #1: apo-ferritin
| Supramolecule | Name: apo-ferritin / type: complex / ID: 1 / Parent: 0 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: OTHER / Digitization - Dimensions - Width: 4096 pixel / Digitization - Dimensions - Height: 4096 pixel / Average electron dose: 19.6 e/Å2 |
| Electron beam | Acceleration voltage: 120 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.6 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller
















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)