+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | Structure of the alpha2A-adrenergic receptor GoA signaling complex bound to oxymetazoline | |||||||||
 マップデータ マップデータ | Structure of Oxymetazoline bound alpha2A-adrenergic receptor GoA signaling complex | |||||||||
 試料 試料 |
| |||||||||
 キーワード キーワード | alpha2A-adrenergic receptor / signaling complex / cryo-EM / MEMBRANE PROTEIN | |||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報negative regulation of uterine smooth muscle contraction / adenylate cyclase-inhibiting adrenergic receptor signaling pathway / phospholipase C-activating adrenergic receptor signaling pathway / alpha2-adrenergic receptor activity / Adrenaline signalling through Alpha-2 adrenergic receptor / alpha-2C adrenergic receptor binding / receptor transactivation / epinephrine binding / alpha-1B adrenergic receptor binding / negative regulation of norepinephrine secretion ...negative regulation of uterine smooth muscle contraction / adenylate cyclase-inhibiting adrenergic receptor signaling pathway / phospholipase C-activating adrenergic receptor signaling pathway / alpha2-adrenergic receptor activity / Adrenaline signalling through Alpha-2 adrenergic receptor / alpha-2C adrenergic receptor binding / receptor transactivation / epinephrine binding / alpha-1B adrenergic receptor binding / negative regulation of norepinephrine secretion / negative regulation of calcium ion transmembrane transporter activity / negative regulation of epinephrine secretion / heterotrimeric G-protein binding / negative regulation of calcium ion-dependent exocytosis / fear response / dopaminergic synapse / Surfactant metabolism / mu-type opioid receptor binding / positive regulation of potassium ion transport / corticotropin-releasing hormone receptor 1 binding / thermoception / thioesterase binding / vesicle docking involved in exocytosis / negative regulation of insulin secretion involved in cellular response to glucose stimulus / positive regulation of membrane protein ectodomain proteolysis / norepinephrine binding / Adrenoceptors / intestinal absorption / positive regulation of epidermal growth factor receptor signaling pathway / G protein-coupled dopamine receptor signaling pathway / regulation of heart contraction / positive regulation of wound healing / activation of protein kinase activity / adrenergic receptor signaling pathway / negative regulation of calcium ion transport / Rho protein signal transduction / regulation of vasoconstriction / negative regulation of lipid catabolic process / negative regulation of insulin secretion / G protein-coupled serotonin receptor binding / adenylate cyclase-activating adrenergic receptor signaling pathway / GABA-ergic synapse / presynaptic active zone membrane / axon terminus / cellular response to hormone stimulus / muscle contraction / presynaptic modulation of chemical synaptic transmission / activation of protein kinase B activity / adenylate cyclase-inhibiting G protein-coupled receptor signaling pathway / guanyl-nucleotide exchange factor activity / positive regulation of cytokine production / female pregnancy / locomotory behavior / postsynaptic density membrane / positive regulation of MAP kinase activity / G-protein beta/gamma-subunit complex binding / Olfactory Signaling Pathway / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / Activation of the phototransduction cascade / adenylate cyclase-activating G protein-coupled receptor signaling pathway / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / G protein-coupled acetylcholine receptor signaling pathway / platelet activation / G-protein activation / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / Prostacyclin signalling through prostacyclin receptor / Glucagon signaling in metabolic regulation / G beta:gamma signalling through CDC42 / G beta:gamma signalling through BTK / ADP signalling through P2Y purinoceptor 12 / Sensory perception of sweet, bitter, and umami (glutamate) taste / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / photoreceptor disc membrane / Glucagon-type ligand receptors / Adrenaline,noradrenaline inhibits insulin secretion / Vasopressin regulates renal water homeostasis via Aquaporins / G alpha (z) signalling events / vasodilation / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / cellular response to catecholamine stimulus / ADORA2B mediated anti-inflammatory cytokines production / sensory perception of taste / ADP signalling through P2Y purinoceptor 1 / G beta:gamma signalling through PI3Kgamma / adenylate cyclase-activating dopamine receptor signaling pathway / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / GPER1 signaling / cellular response to prostaglandin E stimulus / Inactivation, recovery and regulation of the phototransduction cascade / G-protein beta-subunit binding / heterotrimeric G-protein complex / G alpha (12/13) signalling events / extracellular vesicle / signaling receptor complex adaptor activity / Thrombin signalling through proteinase activated receptors (PARs) / GTPase binding / glucose homeostasis 類似検索 - 分子機能 | |||||||||
| 生物種 |  Homo sapiens (ヒト) / Homo sapiens (ヒト) /  | |||||||||
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 3.4 Å | |||||||||
 データ登録者 データ登録者 | Xu J / Cao S | |||||||||
 引用 引用 |  ジャーナル: Sci Adv / 年: 2022 ジャーナル: Sci Adv / 年: 2022タイトル: Structural insights into ligand recognition, activation, and signaling of the α adrenergic receptor. 著者: Jun Xu / Sheng Cao / Harald Hübner / Dorothée Weikert / Geng Chen / Qiuyuan Lu / Daopeng Yuan / Peter Gmeiner / Zheng Liu / Yang Du /   要旨: The α adrenergic receptor (αAR) is a G protein (heterotrimeric guanine nucleotide-binding protein)-coupled receptor that mediates important physiological functions in response to the endogenous ...The α adrenergic receptor (αAR) is a G protein (heterotrimeric guanine nucleotide-binding protein)-coupled receptor that mediates important physiological functions in response to the endogenous neurotransmitters norepinephrine and epinephrine, as well as numerous chemically distinct drugs. However, the molecular mechanisms of drug actions remain poorly understood. Here, we report the cryo-electron microscopy structures of the human αAR-GoA complex bound to norepinephrine and three imidazoline derivatives (brimonidine, dexmedetomidine, and oxymetazoline). Together with mutagenesis and functional data, these structures provide important insights into the molecular basis of ligand recognition, activation, and signaling at the αAR. Further structural analyses uncover different molecular determinants between αAR and βARs for recognition of norepinephrine and key regions that determine the G protein coupling selectivity. Overall, our studies provide a framework for understanding the signal transduction of the adrenergic system at the atomic level, which will facilitate rational structure-based discovery of safer and more effective medications for αAR. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| 添付画像 |
|---|
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_31162.map.gz emd_31162.map.gz | 59.2 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-31162-v30.xml emd-31162-v30.xml emd-31162.xml emd-31162.xml | 16.7 KB 16.7 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| 画像 |  emd_31162.png emd_31162.png | 73 KB | ||
| Filedesc metadata |  emd-31162.cif.gz emd-31162.cif.gz | 6.3 KB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-31162 http://ftp.pdbj.org/pub/emdb/structures/EMD-31162 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-31162 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-31162 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_31162_validation.pdf.gz emd_31162_validation.pdf.gz | 532.9 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_31162_full_validation.pdf.gz emd_31162_full_validation.pdf.gz | 532.5 KB | 表示 | |
| XML形式データ |  emd_31162_validation.xml.gz emd_31162_validation.xml.gz | 6.3 KB | 表示 | |
| CIF形式データ |  emd_31162_validation.cif.gz emd_31162_validation.cif.gz | 7.2 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-31162 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-31162 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-31162 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-31162 | HTTPS FTP |
-関連構造データ
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_31162.map.gz / 形式: CCP4 / 大きさ: 64 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_31162.map.gz / 形式: CCP4 / 大きさ: 64 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | Structure of Oxymetazoline bound alpha2A-adrenergic receptor GoA signaling complex | ||||||||||||||||||||||||||||||||||||
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 0.85 Å | ||||||||||||||||||||||||||||||||||||
| 密度 |
| ||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
|
-添付データ
- 試料の構成要素
試料の構成要素
-全体 : alpha2A-adrenergic receptor Go signaling complex
| 全体 | 名称: alpha2A-adrenergic receptor Go signaling complex |
|---|---|
| 要素 |
|
-超分子 #1: alpha2A-adrenergic receptor Go signaling complex
| 超分子 | 名称: alpha2A-adrenergic receptor Go signaling complex / タイプ: complex / ID: 1 / 親要素: 0 / 含まれる分子: #1-#5 |
|---|
-超分子 #2: alpha2A-adrenergic receptor Go signaling complex
| 超分子 | 名称: alpha2A-adrenergic receptor Go signaling complex / タイプ: complex / ID: 2 / 親要素: 1 / 含まれる分子: #1-#3, #5 |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
-超分子 #3: scFv16
| 超分子 | 名称: scFv16 / タイプ: complex / ID: 3 / 親要素: 1 / 含まれる分子: #4 |
|---|---|
| 由来(天然) | 生物種:  |
-分子 #1: Guanine nucleotide-binding protein G(o) subunit alpha
| 分子 | 名称: Guanine nucleotide-binding protein G(o) subunit alpha タイプ: protein_or_peptide / ID: 1 / コピー数: 1 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 40.1005 KDa |
| 組換発現 | 生物種:  Trichoplusia ni (イラクサキンウワバ) Trichoplusia ni (イラクサキンウワバ) |
| 配列 | 文字列: MGCTLSAEER AALERSKAIE KNLKEDGISA AKDVKLLLLG AGESGKSTIV KQMKIIHEDG FSGEDVKQYK PVVYSNTIQS LAAIVRAMD TLGIEYGDKE RKADAKMVCD VVSRMEDTEP FSAELLSAMM RLWGDSGIQE CFNRSREYQL NDSAKYYLDS L DRIGAADY ...文字列: MGCTLSAEER AALERSKAIE KNLKEDGISA AKDVKLLLLG AGESGKSTIV KQMKIIHEDG FSGEDVKQYK PVVYSNTIQS LAAIVRAMD TLGIEYGDKE RKADAKMVCD VVSRMEDTEP FSAELLSAMM RLWGDSGIQE CFNRSREYQL NDSAKYYLDS L DRIGAADY QPTEQDILRT RVKTTGIVET HFTFKNLHFR LFDVGGQRSE RKKWIHCFED VTAIIFCVAL SGYDQVLHED ET TNRMHES LMLFDSICNN KFFIDTSIIL FLNKKDLFGE KIKKSPLTIC FPEYTGPNTY EDAAAYIQAQ FESKNRSPNK EIY CHMTCA TDTNNIQVVF DAVTDIIIAN NLRGCGLY UniProtKB: Guanine nucleotide-binding protein G(o) subunit alpha |
-分子 #2: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| 分子 | 名称: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 タイプ: protein_or_peptide / ID: 2 / コピー数: 1 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 38.402867 KDa |
| 組換発現 | 生物種:  Trichoplusia ni (イラクサキンウワバ) Trichoplusia ni (イラクサキンウワバ) |
| 配列 | 文字列: HHHHHHGSSG SELDQLRQEA EQLKNQIRDA RKACADATLS QITNNIDPVG RIQMRTRRTL RGHLAKIYAM HWGTDSRLLV SASQDGKLI IWDSYTTNKV HAIPLRSSWV MTCAYAPSGN YVACGGLDNI CSIYNLKTRE GNVRVSRELA GHTGYLSCCR F LDDNQIVT ...文字列: HHHHHHGSSG SELDQLRQEA EQLKNQIRDA RKACADATLS QITNNIDPVG RIQMRTRRTL RGHLAKIYAM HWGTDSRLLV SASQDGKLI IWDSYTTNKV HAIPLRSSWV MTCAYAPSGN YVACGGLDNI CSIYNLKTRE GNVRVSRELA GHTGYLSCCR F LDDNQIVT SSGDTTCALW DIETGQQTTT FTGHTGDVMS LSLAPDTRLF VSGACDASAK LWDVREGMCR QTFTGHESDI NA ICFFPNG NAFATGSDDA TCRLFDLRAD QELMTYSHDN IICGITSVSF SKSGRLLLAG YDDFNCNVWD ALKADRAGVL AGH DNRVSC LGVTDDGMAV ATGSWDSFLK IWN UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 |
-分子 #3: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| 分子 | 名称: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 タイプ: protein_or_peptide / ID: 3 / コピー数: 1 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 7.861143 KDa |
| 組換発現 | 生物種:  Trichoplusia ni (イラクサキンウワバ) Trichoplusia ni (イラクサキンウワバ) |
| 配列 | 文字列: MASNNTASIA QARKLVEQLK MEANIDRIKV SKAAADLMAY CEAHAKEDPL LTPVPASENP FREKKFFCAI L UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 |
-分子 #4: scFv16
| 分子 | 名称: scFv16 / タイプ: protein_or_peptide / ID: 4 / コピー数: 1 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  |
| 分子量 | 理論値: 32.898781 KDa |
| 組換発現 | 生物種:  Trichoplusia ni (イラクサキンウワバ) Trichoplusia ni (イラクサキンウワバ) |
| 配列 | 文字列: MLLVNQSHQG FNKEHTSKMV SAIVLYVLLA AAAHSAFADV QLVESGGGLV QPGGSRKLSC SASGFAFSSF GMHWVRQAPE KGLEWVAYI SSGSGTIYYA DTVKGRFTIS RDDPKNTLFL QMTSLRSEDT AMYYCVRSIY YYGSSPFDFW GQGTTLTVSS G GGGSGGGG ...文字列: MLLVNQSHQG FNKEHTSKMV SAIVLYVLLA AAAHSAFADV QLVESGGGLV QPGGSRKLSC SASGFAFSSF GMHWVRQAPE KGLEWVAYI SSGSGTIYYA DTVKGRFTIS RDDPKNTLFL QMTSLRSEDT AMYYCVRSIY YYGSSPFDFW GQGTTLTVSS G GGGSGGGG SGGGGSDIVM TQATSSVPVT PGESVSISCR SSKSLLHSNG NTYLYWFLQR PGQSPQLLIY RMSNLASGVP DR FSGSGSG TAFTLTISRL EAEDVGVYYC MQHLEYPLTF GAGTKLELKG SLEVLFQGPA AAHHHHHHHH |
-分子 #5: Alpha-2A adrenergic receptor
| 分子 | 名称: Alpha-2A adrenergic receptor / タイプ: protein_or_peptide / ID: 5 / コピー数: 1 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 50.704566 KDa |
| 組換発現 | 生物種:  |
| 配列 | 文字列: MFRQEQPLAE GSFAPMGSLQ PDAGNASWNG TEAPGGGARA TPYSLQVTLT LVCLAGLLML LTVFGNVLVI IAVFTSRALK APQNLFLVS LASADILVAT LVIPFSLANE VMGYWYFGKA WCEIYLALDV LFCTSSIVHL CAISLDRYWS ITQAIEYNLK R TPRRIKAI ...文字列: MFRQEQPLAE GSFAPMGSLQ PDAGNASWNG TEAPGGGARA TPYSLQVTLT LVCLAGLLML LTVFGNVLVI IAVFTSRALK APQNLFLVS LASADILVAT LVIPFSLANE VMGYWYFGKA WCEIYLALDV LFCTSSIVHL CAISLDRYWS ITQAIEYNLK R TPRRIKAI IITVWVISAV ISFPPLISIE KKGGGGGPQP AEPRCEINDQ KWYVISSCIG SFFAPCLIMI LVYVRIYQIA KR RTRVPPS RRGPDAVAAP PGGTERRPNG LGPERSAGPG GAEAEPLPTQ LNGAPGEPAP AGPRDTDALD LEESSSSDHA ERP PGPRRP ERGPRGKGKA RASQVKPGDS LPRRGPGATG IGTPAAGPGE ERVGAAKASR WRGRQNREKR FTFVLAVVIG VFVV CWFPF FFTYTLTAVG CSVPRTLFKF FFWFGYCNSS LNPVIYTIFN HDFRRAFKKI LCRGDRKRIV UniProtKB: Alpha-2A adrenergic receptor |
-分子 #6: Oxymetazoline
| 分子 | 名称: Oxymetazoline / タイプ: ligand / ID: 6 / コピー数: 1 / 式: J5C |
|---|---|
| 分子量 | 理論値: 260.375 Da |
| Chemical component information | 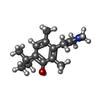 ChemComp-J5C: |
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 緩衝液 | pH: 7.5 |
|---|---|
| 糖包埋 | 材質: vitreous ice |
| 凍結 | 凍結剤: ETHANE |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TITAN KRIOS |
|---|---|
| 撮影 | フィルム・検出器のモデル: GATAN K3 BIOQUANTUM (6k x 4k) 平均電子線量: 56.0 e/Å2 |
| 電子線 | 加速電圧: 300 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 最大 デフォーカス(補正後): 2.0 µm / 最小 デフォーカス(補正後): 0.8 µm / 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD / Cs: 2.7 mm 最大 デフォーカス(公称値): 1.4000000000000001 µm 最小 デフォーカス(公称値): 1.0 µm / 倍率(公称値): 105000 |
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
- 画像解析
画像解析
| 初期モデル | モデルのタイプ: INSILICO MODEL |
|---|---|
| 最終 再構成 | 解像度のタイプ: BY AUTHOR / 解像度: 3.4 Å / 解像度の算出法: FSC 0.143 CUT-OFF / 使用した粒子像数: 220513 |
| 初期 角度割当 | タイプ: RANDOM ASSIGNMENT |
| 最終 角度割当 | タイプ: MAXIMUM LIKELIHOOD |
 ムービー
ムービー コントローラー
コントローラー
































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)




















