[English] 日本語
 Yorodumi
Yorodumi- EMDB-31157: Structure of the alpha2A-adrenergic receptor GoA signaling comple... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of the alpha2A-adrenergic receptor GoA signaling complex bound to dexmedetomidine | |||||||||
 Map data Map data | Structure of dexmedetomidine bound alpha2A-adrenergic receptor GoA signaling complex | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | alpha2A-adrenergic receptor / signaling complex / cryo-EM / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of uterine smooth muscle contraction / alpha2-adrenergic receptor activity / Adrenaline signalling through Alpha-2 adrenergic receptor / adenylate cyclase-inhibiting adrenergic receptor signaling pathway / alpha-2C adrenergic receptor binding / epinephrine binding / phospholipase C-activating adrenergic receptor signaling pathway / alpha-1B adrenergic receptor binding / negative regulation of norepinephrine secretion / negative regulation of epinephrine secretion ...negative regulation of uterine smooth muscle contraction / alpha2-adrenergic receptor activity / Adrenaline signalling through Alpha-2 adrenergic receptor / adenylate cyclase-inhibiting adrenergic receptor signaling pathway / alpha-2C adrenergic receptor binding / epinephrine binding / phospholipase C-activating adrenergic receptor signaling pathway / alpha-1B adrenergic receptor binding / negative regulation of norepinephrine secretion / negative regulation of epinephrine secretion / heterotrimeric G-protein binding / negative regulation of calcium ion-dependent exocytosis / Surfactant metabolism / positive regulation of potassium ion transport / dopaminergic synapse / thermoception / mu-type opioid receptor binding / fear response / corticotropin-releasing hormone receptor 1 binding / thioesterase binding / negative regulation of insulin secretion involved in cellular response to glucose stimulus / norepinephrine binding / positive regulation of membrane protein ectodomain proteolysis / vesicle docking involved in exocytosis / Adrenoceptors / G protein-coupled dopamine receptor signaling pathway / response to alcohol / intestinal absorption / adrenergic receptor signaling pathway / positive regulation of wound healing / response to morphine / regulation of heart contraction / negative regulation of calcium ion transport / parallel fiber to Purkinje cell synapse / negative regulation of lipid catabolic process / regulation of vasoconstriction / positive regulation of epidermal growth factor receptor signaling pathway / postsynaptic modulation of chemical synaptic transmission / cellular response to hormone stimulus / Rho protein signal transduction / adenylate cyclase-activating adrenergic receptor signaling pathway / presynaptic active zone membrane / G protein-coupled serotonin receptor binding / adenylate cyclase regulator activity / adenylate cyclase-inhibiting serotonin receptor signaling pathway / presynaptic modulation of chemical synaptic transmission / axon terminus / muscle contraction / guanyl-nucleotide exchange factor activity / positive regulation of cytokine production / locomotory behavior / female pregnancy / negative regulation of insulin secretion / platelet activation / postsynaptic density membrane / adenylate cyclase-inhibiting G protein-coupled receptor signaling pathway / vasodilation / GABA-ergic synapse / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / G-protein beta/gamma-subunit complex binding / adenylate cyclase-activating G protein-coupled receptor signaling pathway / Olfactory Signaling Pathway / Activation of the phototransduction cascade / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / G protein-coupled acetylcholine receptor signaling pathway / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / G-protein activation / G beta:gamma signalling through CDC42 / Prostacyclin signalling through prostacyclin receptor / Glucagon signaling in metabolic regulation / G beta:gamma signalling through BTK / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / ADP signalling through P2Y purinoceptor 12 / photoreceptor disc membrane / Glucagon-type ligand receptors / Sensory perception of sweet, bitter, and umami (glutamate) taste / Adrenaline,noradrenaline inhibits insulin secretion / Vasopressin regulates renal water homeostasis via Aquaporins / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / G alpha (z) signalling events / ADP signalling through P2Y purinoceptor 1 / ADORA2B mediated anti-inflammatory cytokines production / cellular response to catecholamine stimulus / G beta:gamma signalling through PI3Kgamma / adenylate cyclase-activating dopamine receptor signaling pathway / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / GPER1 signaling / G-protein beta-subunit binding / glucose homeostasis / cellular response to prostaglandin E stimulus / heterotrimeric G-protein complex / G alpha (12/13) signalling events / Inactivation, recovery and regulation of the phototransduction cascade / extracellular vesicle / sensory perception of taste / Thrombin signalling through proteinase activated receptors (PARs) / signaling receptor complex adaptor activity Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.6 Å | |||||||||
 Authors Authors | Xu J / Cao S | |||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2022 Journal: Sci Adv / Year: 2022Title: Structural insights into ligand recognition, activation, and signaling of the α adrenergic receptor. Authors: Jun Xu / Sheng Cao / Harald Hübner / Dorothée Weikert / Geng Chen / Qiuyuan Lu / Daopeng Yuan / Peter Gmeiner / Zheng Liu / Yang Du /   Abstract: The α adrenergic receptor (αAR) is a G protein (heterotrimeric guanine nucleotide-binding protein)-coupled receptor that mediates important physiological functions in response to the endogenous ...The α adrenergic receptor (αAR) is a G protein (heterotrimeric guanine nucleotide-binding protein)-coupled receptor that mediates important physiological functions in response to the endogenous neurotransmitters norepinephrine and epinephrine, as well as numerous chemically distinct drugs. However, the molecular mechanisms of drug actions remain poorly understood. Here, we report the cryo-electron microscopy structures of the human αAR-GoA complex bound to norepinephrine and three imidazoline derivatives (brimonidine, dexmedetomidine, and oxymetazoline). Together with mutagenesis and functional data, these structures provide important insights into the molecular basis of ligand recognition, activation, and signaling at the αAR. Further structural analyses uncover different molecular determinants between αAR and βARs for recognition of norepinephrine and key regions that determine the G protein coupling selectivity. Overall, our studies provide a framework for understanding the signal transduction of the adrenergic system at the atomic level, which will facilitate rational structure-based discovery of safer and more effective medications for αAR. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_31157.map.gz emd_31157.map.gz | 59.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-31157-v30.xml emd-31157-v30.xml emd-31157.xml emd-31157.xml | 16.7 KB 16.7 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_31157.png emd_31157.png | 88.3 KB | ||
| Filedesc metadata |  emd-31157.cif.gz emd-31157.cif.gz | 6.4 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-31157 http://ftp.pdbj.org/pub/emdb/structures/EMD-31157 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-31157 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-31157 | HTTPS FTP |
-Related structure data
| Related structure data |  7ejaMC  7ej0C  7ej8C  7ejkC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_31157.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_31157.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Structure of dexmedetomidine bound alpha2A-adrenergic receptor GoA signaling complex | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.85 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : alpha2A-adrenergic receptor Go signaling complex
| Entire | Name: alpha2A-adrenergic receptor Go signaling complex |
|---|---|
| Components |
|
-Supramolecule #1: alpha2A-adrenergic receptor Go signaling complex
| Supramolecule | Name: alpha2A-adrenergic receptor Go signaling complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#5 |
|---|
-Supramolecule #2: alpha2A-adrenergic receptor Go signaling complex
| Supramolecule | Name: alpha2A-adrenergic receptor Go signaling complex / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1-#4 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Supramolecule #3: scFv16
| Supramolecule | Name: scFv16 / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #5 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Guanine nucleotide-binding protein G(o) subunit alpha
| Macromolecule | Name: Guanine nucleotide-binding protein G(o) subunit alpha / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 40.1005 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MGCTLSAEER AALERSKAIE KNLKEDGISA AKDVKLLLLG AGESGKSTIV KQMKIIHEDG FSGEDVKQYK PVVYSNTIQS LAAIVRAMD TLGIEYGDKE RKADAKMVCD VVSRMEDTEP FSAELLSAMM RLWGDSGIQE CFNRSREYQL NDSAKYYLDS L DRIGAADY ...String: MGCTLSAEER AALERSKAIE KNLKEDGISA AKDVKLLLLG AGESGKSTIV KQMKIIHEDG FSGEDVKQYK PVVYSNTIQS LAAIVRAMD TLGIEYGDKE RKADAKMVCD VVSRMEDTEP FSAELLSAMM RLWGDSGIQE CFNRSREYQL NDSAKYYLDS L DRIGAADY QPTEQDILRT RVKTTGIVET HFTFKNLHFR LFDVGGQRSE RKKWIHCFED VTAIIFCVAL SGYDQVLHED ET TNRMHES LMLFDSICNN KFFIDTSIIL FLNKKDLFGE KIKKSPLTIC FPEYTGPNTY EDAAAYIQAQ FESKNRSPNK EIY CHMTCA TDTNNIQVVF DAVTDIIIAN NLRGCGLY UniProtKB: Guanine nucleotide-binding protein G(o) subunit alpha |
-Macromolecule #2: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 38.402867 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: HHHHHHGSSG SELDQLRQEA EQLKNQIRDA RKACADATLS QITNNIDPVG RIQMRTRRTL RGHLAKIYAM HWGTDSRLLV SASQDGKLI IWDSYTTNKV HAIPLRSSWV MTCAYAPSGN YVACGGLDNI CSIYNLKTRE GNVRVSRELA GHTGYLSCCR F LDDNQIVT ...String: HHHHHHGSSG SELDQLRQEA EQLKNQIRDA RKACADATLS QITNNIDPVG RIQMRTRRTL RGHLAKIYAM HWGTDSRLLV SASQDGKLI IWDSYTTNKV HAIPLRSSWV MTCAYAPSGN YVACGGLDNI CSIYNLKTRE GNVRVSRELA GHTGYLSCCR F LDDNQIVT SSGDTTCALW DIETGQQTTT FTGHTGDVMS LSLAPDTRLF VSGACDASAK LWDVREGMCR QTFTGHESDI NA ICFFPNG NAFATGSDDA TCRLFDLRAD QELMTYSHDN IICGITSVSF SKSGRLLLAG YDDFNCNVWD ALKADRAGVL AGH DNRVSC LGVTDDGMAV ATGSWDSFLK IWN UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 |
-Macromolecule #3: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 7.861143 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MASNNTASIA QARKLVEQLK MEANIDRIKV SKAAADLMAY CEAHAKEDPL LTPVPASENP FREKKFFCAI L UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 |
-Macromolecule #4: Alpha-2A adrenergic receptor
| Macromolecule | Name: Alpha-2A adrenergic receptor / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 50.704566 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MFRQEQPLAE GSFAPMGSLQ PDAGNASWNG TEAPGGGARA TPYSLQVTLT LVCLAGLLML LTVFGNVLVI IAVFTSRALK APQNLFLVS LASADILVAT LVIPFSLANE VMGYWYFGKA WCEIYLALDV LFCTSSIVHL CAISLDRYWS ITQAIEYNLK R TPRRIKAI ...String: MFRQEQPLAE GSFAPMGSLQ PDAGNASWNG TEAPGGGARA TPYSLQVTLT LVCLAGLLML LTVFGNVLVI IAVFTSRALK APQNLFLVS LASADILVAT LVIPFSLANE VMGYWYFGKA WCEIYLALDV LFCTSSIVHL CAISLDRYWS ITQAIEYNLK R TPRRIKAI IITVWVISAV ISFPPLISIE KKGGGGGPQP AEPRCEINDQ KWYVISSCIG SFFAPCLIMI LVYVRIYQIA KR RTRVPPS RRGPDAVAAP PGGTERRPNG LGPERSAGPG GAEAEPLPTQ LNGAPGEPAP AGPRDTDALD LEESSSSDHA ERP PGPRRP ERGPRGKGKA RASQVKPGDS LPRRGPGATG IGTPAAGPGE ERVGAAKASR WRGRQNREKR FTFVLAVVIG VFVV CWFPF FFTYTLTAVG CSVPRTLFKF FFWFGYCNSS LNPVIYTIFN HDFRRAFKKI LCRGDRKRIV UniProtKB: Alpha-2A adrenergic receptor |
-Macromolecule #5: scFv16
| Macromolecule | Name: scFv16 / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 32.898781 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MLLVNQSHQG FNKEHTSKMV SAIVLYVLLA AAAHSAFADV QLVESGGGLV QPGGSRKLSC SASGFAFSSF GMHWVRQAPE KGLEWVAYI SSGSGTIYYA DTVKGRFTIS RDDPKNTLFL QMTSLRSEDT AMYYCVRSIY YYGSSPFDFW GQGTTLTVSS G GGGSGGGG ...String: MLLVNQSHQG FNKEHTSKMV SAIVLYVLLA AAAHSAFADV QLVESGGGLV QPGGSRKLSC SASGFAFSSF GMHWVRQAPE KGLEWVAYI SSGSGTIYYA DTVKGRFTIS RDDPKNTLFL QMTSLRSEDT AMYYCVRSIY YYGSSPFDFW GQGTTLTVSS G GGGSGGGG SGGGGSDIVM TQATSSVPVT PGESVSISCR SSKSLLHSNG NTYLYWFLQR PGQSPQLLIY RMSNLASGVP DR FSGSGSG TAFTLTISRL EAEDVGVYYC MQHLEYPLTF GAGTKLELKG SLEVLFQGPA AAHHHHHHHH |
-Macromolecule #6: 4-[(1~{S})-1-(2,3-dimethylphenyl)ethyl]-1~{H}-imidazole
| Macromolecule | Name: 4-[(1~{S})-1-(2,3-dimethylphenyl)ethyl]-1~{H}-imidazole type: ligand / ID: 6 / Number of copies: 1 / Formula: CZX |
|---|---|
| Molecular weight | Theoretical: 200.28 Da |
| Chemical component information | 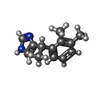 ChemComp-CZX: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Sugar embedding | Material: vitreous ice |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 56.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated defocus max: 2.0 µm / Calibrated defocus min: 0.8 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 1.4000000000000001 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 105000 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: INSILICO MODEL |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.6 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 455506 |
| Initial angle assignment | Type: RANDOM ASSIGNMENT |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller



























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)




















