+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of LRP2 at pH 5.2 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | LRP2 / Megalin / GP330 / Endocytosis / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationTransport of RCbl within the body / endocytic hemoglobin import into cell / chemoattraction of axon / diol metabolic process / positive regulation of lipoprotein transport / pulmonary artery morphogenesis / secondary heart field specification / Retinoid metabolism and transport / positive regulation of oligodendrocyte progenitor proliferation / folate import across plasma membrane ...Transport of RCbl within the body / endocytic hemoglobin import into cell / chemoattraction of axon / diol metabolic process / positive regulation of lipoprotein transport / pulmonary artery morphogenesis / secondary heart field specification / Retinoid metabolism and transport / positive regulation of oligodendrocyte progenitor proliferation / folate import across plasma membrane / ventricular compact myocardium morphogenesis / metal ion transport / response to leptin / protein transporter activity / hormone binding / Cargo recognition for clathrin-mediated endocytosis / vitamin D metabolic process / neuron projection arborization / Clathrin-mediated endocytosis / coronary artery morphogenesis / outflow tract septum morphogenesis / coronary vasculature development / insulin-like growth factor I binding / transcytosis / protein import / aorta development / ventricular septum development / positive regulation of neurogenesis / endosomal transport / low-density lipoprotein particle receptor binding / hemoglobin binding / brush border / vagina development / positive regulation of endocytosis / amyloid-beta clearance / endocytic vesicle / response to X-ray / negative regulation of BMP signaling pathway / axonal growth cone / clathrin-coated pit / forebrain development / phosphatidylinositol 3-kinase/protein kinase B signal transduction / receptor-mediated endocytosis / kidney development / neural tube closure / endosome lumen / nuclear receptor binding / PDZ domain binding / brush border membrane / sensory perception of sound / cellular response to growth factor stimulus / SH3 domain binding / endocytosis / male gonad development / protein transport / apical part of cell / heart development / protein-folding chaperone binding / cell population proliferation / receptor complex / endosome / apical plasma membrane / axon / external side of plasma membrane / dendrite / calcium ion binding / protein-containing complex binding / negative regulation of apoptotic process / Golgi apparatus / cell surface / endoplasmic reticulum / protein-containing complex / extracellular space / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.97 Å | |||||||||
 Authors Authors | Beenken A / Cerutti G / Fitzpatrick AW / Barasch J / Shapiro L | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Cell / Year: 2023 Journal: Cell / Year: 2023Title: Structures of LRP2 reveal a molecular machine for endocytosis. Authors: Andrew Beenken / Gabriele Cerutti / Julia Brasch / Yicheng Guo / Zizhang Sheng / Hediye Erdjument-Bromage / Zainab Aziz / Shelief Y Robbins-Juarez / Estefania Y Chavez / Goran Ahlsen / ...Authors: Andrew Beenken / Gabriele Cerutti / Julia Brasch / Yicheng Guo / Zizhang Sheng / Hediye Erdjument-Bromage / Zainab Aziz / Shelief Y Robbins-Juarez / Estefania Y Chavez / Goran Ahlsen / Phinikoula S Katsamba / Thomas A Neubert / Anthony W P Fitzpatrick / Jonathan Barasch / Lawrence Shapiro /  Abstract: The low-density lipoprotein (LDL) receptor-related protein 2 (LRP2 or megalin) is representative of the phylogenetically conserved subfamily of giant LDL receptor-related proteins, which function in ...The low-density lipoprotein (LDL) receptor-related protein 2 (LRP2 or megalin) is representative of the phylogenetically conserved subfamily of giant LDL receptor-related proteins, which function in endocytosis and are implicated in diseases of the kidney and brain. Here, we report high-resolution cryoelectron microscopy structures of LRP2 isolated from mouse kidney, at extracellular and endosomal pH. The structures reveal LRP2 to be a molecular machine that adopts a conformation for ligand binding at the cell surface and for ligand shedding in the endosome. LRP2 forms a homodimer, the conformational transformation of which is governed by pH-sensitive sites at both homodimer and intra-protomer interfaces. A subset of LRP2 deleterious missense variants in humans appears to impair homodimer assembly. These observations lay the foundation for further understanding the function and mechanism of LDL receptors and implicate homodimerization as a conserved feature of the LRP receptor subfamily. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_28241.map.gz emd_28241.map.gz | 254.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-28241-v30.xml emd-28241-v30.xml emd-28241.xml emd-28241.xml | 24.9 KB 24.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_28241_fsc.xml emd_28241_fsc.xml | 16.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_28241.png emd_28241.png | 94.7 KB | ||
| Filedesc metadata |  emd-28241.cif.gz emd-28241.cif.gz | 8.5 KB | ||
| Others |  emd_28241_additional_1.map.gz emd_28241_additional_1.map.gz emd_28241_additional_2.map.gz emd_28241_additional_2.map.gz emd_28241_half_map_1.map.gz emd_28241_half_map_1.map.gz emd_28241_half_map_2.map.gz emd_28241_half_map_2.map.gz | 28.7 MB 242.2 MB 474.6 MB 474.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-28241 http://ftp.pdbj.org/pub/emdb/structures/EMD-28241 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28241 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28241 | HTTPS FTP |
-Validation report
| Summary document |  emd_28241_validation.pdf.gz emd_28241_validation.pdf.gz | 983.4 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_28241_full_validation.pdf.gz emd_28241_full_validation.pdf.gz | 983 KB | Display | |
| Data in XML |  emd_28241_validation.xml.gz emd_28241_validation.xml.gz | 26 KB | Display | |
| Data in CIF |  emd_28241_validation.cif.gz emd_28241_validation.cif.gz | 34.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-28241 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-28241 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-28241 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-28241 | HTTPS FTP |
-Related structure data
| Related structure data |  8em7MC  8em4C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_28241.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_28241.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.83 Å | ||||||||||||||||||||||||||||||||||||
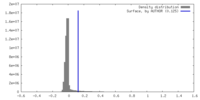
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: Composite of unsharpened local refinement maps
| File | emd_28241_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Composite of unsharpened local refinement maps | ||||||||||||
| Projections & Slices |
| ||||||||||||
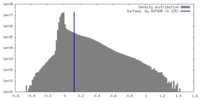
| Density Histograms |
-Additional map: Composite of global map with deepEMhancer density-modified local...
| File | emd_28241_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Composite of global map with deepEMhancer density-modified local refinements | ||||||||||||
| Projections & Slices |
| ||||||||||||
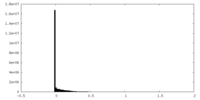
| Density Histograms |
-Half map: #2
| File | emd_28241_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
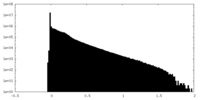
| Density Histograms |
-Half map: #1
| File | emd_28241_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : LRP2 at endosomal pH
| Entire | Name: LRP2 at endosomal pH |
|---|---|
| Components |
|
-Supramolecule #1: LRP2 at endosomal pH
| Supramolecule | Name: LRP2 at endosomal pH / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 / Details: Endogenously purified from mouse kidney |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Low-density lipoprotein receptor-related protein 2
| Macromolecule | Name: Low-density lipoprotein receptor-related protein 2 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 519.7465 KDa |
| Sequence | String: MERGAAAAAW MLLLAIAACL APVSGQECGS GNFRCDNGYC IPASWRCDGT RDCLDDTDEI GCPPRSCGSG FFLCPAEGTC IPSSWVCDQ DKDCSDGADE QQNCPGTTCS SQQLTCSNGQ CVPIEYRCDH VSDCPDGSDE RNCYYPTCDQ LTCANGACYN T SQKCDHKV ...String: MERGAAAAAW MLLLAIAACL APVSGQECGS GNFRCDNGYC IPASWRCDGT RDCLDDTDEI GCPPRSCGSG FFLCPAEGTC IPSSWVCDQ DKDCSDGADE QQNCPGTTCS SQQLTCSNGQ CVPIEYRCDH VSDCPDGSDE RNCYYPTCDQ LTCANGACYN T SQKCDHKV DCRDSSDEAN CTTLCSQKEF QCGSGECILR AYVCDHDNDC EDNSDEHNCN YDTCGGHQFT CSNGQCINQN WV CDGDDDC QDSGDEDGCE SNQRHHTCYP REWACPGSGR CISMDKVCDG VPDCPEGEDE NNATSGRYCG TGLCSILNCE YQC HQTPYG GECFCPPGHI INSNDSRTCI DFDDCQIWGI CDQKCESRQG RHQCLCEEGY ILERGQHCKS NDSFSAASII FSNG RDLLV GDLHGRNFRI LAESKNRGIV MGVDFHYQKH RVFWTDPMQA KVFSTDINGL NTQEILNVSI DAPENLAVDW INNKL YLVE TRVNRIDVVN LEGNQRVTLI TENLGHPRGI ALDPTVGYLF FSDWGSLSGQ PKVERAFMDG SNRKDLVTTK LGWPAG ITL DLVSKRVYWV DSRYDYIETV TYDGIQRKTV ARGGSLVPHP FGISLFEEHV FFTDWTKMAV MKANKFTDTN PQVYHQS SL TPFGVTVYHA LRQPNATNPC GNNNGGCAQI CVLSHRTDNG GLGYRCKCEF GFELDADEHH CVAVKNFLLF SSQTAVRG I PFTLSTQEDV MVPVTGSPSF FVGIDFDAQH STIFYSDLSK NIIYQQKIDG TGKEVITANR LQNVECLSFD WISRNLYWT DGGSKSVTVM KLADKSRRQI ISNLNNPRSI VVHPAAGYMF LSDWFRPAKI MRAWSDGSHL MPIVNTSLGW PNGLAIDWST SRLYWVDAF FDKIEHSNLD GLDRKRLGHV DQMTHPFGLT VFKDNVFLTD WRLGAIIRVR KSDGGDMTVV RRGISSIMHV K AYDADLQT GTNYCSQTTH PNGDCSHFCF PVPNFQRVCG CPYGMKLQRD QMTCEGDPAR EPPTQQCGSS SFPCNNGKCV PS IFRCDGV DDCHDNSDEH QCGALNNTCS SSAFTCVHGG QCIPGQWRCD KQNDCLDGSD EQNCPTRSPS STCPPTSFTC DNH MCIPKE WVCDTDNDCS DGSDEKNCQA SGTCHPTQFR CPDHRCISPL YVCDGDKDCV DGSDEAGCVL NCTSSQFKCA DGSS CINSR YRCDGVYDCK DNSDEAGCPT RPPGMCHPDE FQCQGDGTCI PNTWECDGHP DCIQGSDEHN GCVPKTCSPS HFLCD NGNC IYNSWVCDGD NDCRDMSDEK DCPTQPFHCP SSQWQCPGYS ICVNLSALCD GVFDCPNGTD ESPLCNQDSC LHFNGG CTH RCIQGPFGAT CVCPIGYQLA NDTKTCEDVN ECDIPGFCSQ HCVNMRGSFR CACDPEYTLE SDGRTCKVTA SENLLLV VA SRDKIIMDNI TAHTHNIYSL VQDVSFVVAL DFDSVTGRVF WSDLLEGKTW SAFQNGTDKR VVHDSGLSLT EMIAVDWI G RNIYWTDYTL ETIEVSKIDG SHRTVLISKN VTKPRGLALD PRMGDNVMFW SDWGHHPRIE RASMDGTMRT VIVQEKIYW PCGLSIDYPN RLIYFMDAYL DYIEFCDYDG QNRRQVIASD LVLHHPHALT LFEDSVFWTD RGTHQVMQAN KWHGRNQSVV MYSVPQPLG IIAIHPSRQP SSPNPCASAT CSHLCLLSAQ EPRHYSCACP SGWNLSDDSV NCVRGDQPFL ISVRENVIFG I SLDPEVKS NDAMVPISGI QHGYDVEFDD SEQFIYWVEN PGEIHRVKTD GSNRTAFAPL SLLGSSLGLA LDWVSRNIYY TT PASRSIE VLTLRGDTRY GKTLITNDGT PLGVGFPVGI AVDPARGKLY WSDHGTDSGV PAKIASANMD GTSLKILFTG NME HLEVVT LDIQEQKLYW AVTSRGVIER GNVDGTERMI LVHHLAHPWG LVVHGSFLYY SDEQYEVIER VDKSSGSNKV VFRD NIPYL RGLRVYHHRN AADSSNGCSN NPNACQQICL PVPGGMFSCA CASGFKLSPD GRSCSPYNSF IVVSMLPAVR GFSLE LSDH SEAMVPVAGQ GRNVLHADVD VANGFIYWCD FSSSVRSSNG IRRIKPNGSN FTNIVTYGIG ANGIRGVAVD WVAGNL YFT NAFVYETLIE VIRINTTYRR VLLKVSVDMP RHIVVDPKHR YLFWADYGQK PKIERSFLDC TNRTVLVSEG IVTPRGL AV DHDTGYIYWV DDSLDIIARI HRDGGESQVV RYGSRYPTPY GITVFGESII WVDRNLRKVF QASKQPGNTD PPTVIRDS I NLLRDVTIFD EHVQPLSPAE LNNNPCLQSN GGCSHFCFAL PELPTPKCGC AFGTLEDDGK NCATSREDFL IYSLNNSLR SLHFDPQDHN LPFQAISVEG MAIALDYDRR NNRIFFTQKL NPIRGQISYV NLYSGASSPT ILLSNIGVTD GIAFDWINRR IYYSDFSNQ TINSMAEDGS NRAVIARVSK PRAIVLDPCR GYMYWTDWGT NAKIERATLG GNFRVPIVNT SLVWPNGLTL D LETDLLYW ADASLQKIER STLTGSNREV VISTAFHSFG LTVYGQYIYW TDFYTKKIYR ANKYDGSDLI AMTTRLPTQP SG ISTVVKT QQQQCSNPCD QFNGGCSHIC APGPNGAECQ CPHEGSWYLA NDNKYCVVDT GARCNQFQFT CLNGRCISQD WKC DNDNDC GDGSDELPTV CAFHTCRSTA FTCANGRCVP YHYRCDFYND CGDNSDEAGC LFRSCNSTTE FTCSNGRCIP LSYV CNGIN NCHDNDTSDE KNCPPITCQP DFAKCQTTNI CVPRAFLCDG DNDCGDGSDE NPIYCASHTC RSNEFQCVSP HRCIP SYWF CDGEADCVDS SDEPDTCGHS LNSCSANQFH CDNGRCISSS WVCDGDNDCG DMSDEDQRHH CELQNCSSTE FTCINS RPP NRRCIPQHWV CDGDADCADA LDELQNCTMR ACSTGEFSCA NGRCIRQSFR CDRRNDCGDY SDERGCSYPP CRDDQFT CQ NGQCITKLYV CDEDNDCGDG SDEQEHLCHT PEPTCPPHQF RCDNGHCIEM GTVCNHVDDC SDNSDEKGCG INECQDSS I SHCDHNCTDT ITSFYCSCLP GYKLMSDKRT CVDIDECKET PQLCSQKCEN VIGSYICKCA PGYIREPDGK SCRQNSNIE PYLVFSNRYY IRNLTIDGTS YSLILQGLGN VVALDFDRVE ERLYWIDAEK QIIERMFLNK TNQETIISHR LRRAESLAVD WVSRKLYWL DAILDCLFVS DLEGRQRKML AQHCVDANNT FCFENPRGIV LHPQRGYVYW ADWGDHAYIA RIGMDGTNKT V IISTKIEW PNAITIDYTN DLLYWADAHL GYIEFSDLEG HHRHTVYDGT LPHPFALTIF EDTVFWTDWN TRTVEKGNKY DG SGRVVLV NTTHKPFDIH VLHPYRQPIM SNPCATNNGG CSHLCLIKAG GRGFTCECPD DFQTVQLRDR TLCMPMCSST QFL CGNNEK CIPIWWKCDG QKDCSDGSDE SDLCPHRFCR LGQFQCRDGN CTSPQALCNA RQDCADGSDE DRVLCEHHRC EANE WQCAN KRCIPEYWQC DSVDDCLDNS DEDPSHCASR TCRPGQFKCN NGRCIPQSWK CDVDNDCGDY SDEPIHECMT AAYNC DNHT EFSCKTNYRC IPQWAVCNGF DDCRDNSDEQ GCESVPCHPS GDFRCGNHHC IPLRWKCDGI DDCGDNSDEE SCVPRE CTE SEFRCADQQC IPSRWVCDQE NDCGDNSDER DCEMKTCHPE HFQCTSGHCV PKALACDGRA DCLDASDESA CPTRFPN GT YCPAAMFECK NHVCIQSFWI CDGENDCVDG SDEEIHLCFN VPCESPQRFR CDNSRCIYGH QLCNGVDDCG DGSDEKEE H CRKPTHKPCT DTEYKCSNGN CVSQHYVCDN VDDCGDLSDE TGCNLGENRT CAEKICEQNC TQLSNGGFIC SCRPGFKPS TLDKNSCQDI NECEEFGICP QSCRNSKGSY ECFCVDGFKS MSTHYGERCA ADGSPPLLLL PENVRIRKYN ISSEKFSEYL EEEEHIQAI DYDWDPEGIG LSVVYYTVLS QGSQFGAIKR AYLPDFESGS NNPVREVDLG LKYLMQPDGL AVDWVGRHIY W SDAKSQRI EVATLDGRYR KWLITTQLDQ PAAIAVNPKL GLMFWTDQGK QPKIESAWMN GEHRSVLASA NLGWPNGLSI DY LNGDRIY WSDSKEDVIE SIKYDGTDRR LIINDAMKPF SLDIFEDQLY WVAKEKGEVW RQNKFGKGNK EKLLVVNPWL TQV RIFHQL RYNQSVSNPC KQVCSHLCLL RPGGYSCACP QGSDFVTGST VECDAASELP ITMPSPCRCM HGGSCYFDEN DLPK CKCSS GYSGEYCEIG LSRGIPPGTT MALLLTFAMV IIVGALVLVG FFHYRKTGSL LPSLPKLPSL SSLAKPSENG NGVTF RSGA DVNMDIGVSP FGPETIIDRS MAMNEQFVME VGKQPVIFEN PMYAAKDSTS KVGLAVQGPS VSSQVTVPEN VENQNY GRS IDPSEIVPEP KPASPGADET QGTKWNIFKR KPKQTTNFEN PIYAEMDTEQ KEAVAVAPPP SPSLPAKASK RSSTPGY TA TEDTFKDTAN LVKEDSDV UniProtKB: Low-density lipoprotein receptor-related protein 2 |
-Macromolecule #3: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 3 / Number of copies: 78 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Macromolecule #4: 2-acetamido-2-deoxy-beta-D-galactopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-galactopyranose / type: ligand / ID: 4 / Number of copies: 44 / Formula: NGA |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NGA: |
-Macromolecule #5: CALCIUM ION
| Macromolecule | Name: CALCIUM ION / type: ligand / ID: 5 / Number of copies: 88 / Formula: CA |
|---|---|
| Molecular weight | Theoretical: 40.078 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 2 mg/mL |
|---|---|
| Buffer | pH: 5.2 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 58.06 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.5 µm / Nominal defocus min: 0.5 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Protocol: FLEXIBLE FIT |
|---|---|
| Output model |  PDB-8em7: |
 Movie
Movie Controller
Controller

























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)