[English] 日本語
 Yorodumi
Yorodumi- EMDB-2752: Structure of the ryanodine receptor at resolution of 8.5 A in par... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-2752 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of the ryanodine receptor at resolution of 8.5 A in partially open state | |||||||||
 Map data Map data | reconstruction of ryanodine receptor 1 in partially open state | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | calcium binding / ion channel / muscular contraction / conformational changes. | |||||||||
| Function / homology |  Function and homology information Function and homology informationATP-gated ion channel activity / terminal cisterna / ryanodine receptor complex / ryanodine-sensitive calcium-release channel activity / release of sequestered calcium ion into cytosol by sarcoplasmic reticulum / ossification involved in bone maturation / skin development / organelle membrane / cellular response to caffeine / intracellularly gated calcium channel activity ...ATP-gated ion channel activity / terminal cisterna / ryanodine receptor complex / ryanodine-sensitive calcium-release channel activity / release of sequestered calcium ion into cytosol by sarcoplasmic reticulum / ossification involved in bone maturation / skin development / organelle membrane / cellular response to caffeine / intracellularly gated calcium channel activity / outflow tract morphogenesis / toxic substance binding / smooth endoplasmic reticulum / striated muscle contraction / voltage-gated calcium channel activity / skeletal muscle fiber development / muscle contraction / sarcoplasmic reticulum membrane / release of sequestered calcium ion into cytosol / cellular response to calcium ion / sarcoplasmic reticulum / sarcolemma / calcium ion transmembrane transport / calcium channel activity / Z disc / intracellular calcium ion homeostasis / disordered domain specific binding / protein homotetramerization / transmembrane transporter binding / calmodulin binding / calcium ion binding / ATP binding / identical protein binding / membrane Similarity search - Function | |||||||||
| Biological species |  | |||||||||
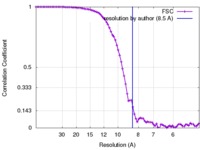
| Method | single particle reconstruction / cryo EM / Resolution: 8.5 Å | |||||||||
 Authors Authors | Efremov RG / Leitner A / Aebersold R / Raunser S | |||||||||
 Citation Citation |  Journal: Nature / Year: 2015 Journal: Nature / Year: 2015Title: Architecture and conformational switch mechanism of the ryanodine receptor. Authors: Rouslan G Efremov / Alexander Leitner / Ruedi Aebersold / Stefan Raunser /    Abstract: Muscle contraction is initiated by the release of calcium (Ca(2+)) from the sarcoplasmic reticulum into the cytoplasm of myocytes through ryanodine receptors (RyRs). RyRs are homotetrameric channels ...Muscle contraction is initiated by the release of calcium (Ca(2+)) from the sarcoplasmic reticulum into the cytoplasm of myocytes through ryanodine receptors (RyRs). RyRs are homotetrameric channels with a molecular mass of more than 2.2 megadaltons that are regulated by several factors, including ions, small molecules and proteins. Numerous mutations in RyRs have been associated with human diseases. The molecular mechanism underlying the complex regulation of RyRs is poorly understood. Using electron cryomicroscopy, here we determine the architecture of rabbit RyR1 at a resolution of 6.1 Å. We show that the cytoplasmic moiety of RyR1 contains two large α-solenoid domains and several smaller domains, with folds suggestive of participation in protein-protein interactions. The transmembrane domain represents a chimaera of voltage-gated sodium and pH-activated ion channels. We identify the calcium-binding EF-hand domain and show that it functions as a conformational switch allosterically gating the channel. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_2752.map.gz emd_2752.map.gz | 2.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-2752-v30.xml emd-2752-v30.xml emd-2752.xml emd-2752.xml | 12.3 KB 12.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_2752_fsc.xml emd_2752_fsc.xml | 7.5 KB | Display |  FSC data file FSC data file |
| Images |  emd_2752.jpg emd_2752.jpg | 50 KB | ||
| Masks |  emd_2752_msk_1.map emd_2752_msk_1.map | 42.9 MB |  Mask map Mask map | |
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-2752 http://ftp.pdbj.org/pub/emdb/structures/EMD-2752 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2752 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2752 | HTTPS FTP |
-Validation report
| Summary document |  emd_2752_validation.pdf.gz emd_2752_validation.pdf.gz | 290.9 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_2752_full_validation.pdf.gz emd_2752_full_validation.pdf.gz | 290 KB | Display | |
| Data in XML |  emd_2752_validation.xml.gz emd_2752_validation.xml.gz | 10 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-2752 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-2752 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-2752 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-2752 | HTTPS FTP |
-Related structure data
| Related structure data |  4uweMC  2751C  4uwaC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_2752.map.gz / Format: CCP4 / Size: 41.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_2752.map.gz / Format: CCP4 / Size: 41.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | reconstruction of ryanodine receptor 1 in partially open state | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 2.59 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Segmentation: mask used for calculating FSC curve
| Annotation | mask used for calculating FSC curve | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| File |  emd_2752_msk_1.map emd_2752_msk_1.map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Ryanodine receptor 1 (calcium release channel) from rabbit
| Entire | Name: Ryanodine receptor 1 (calcium release channel) from rabbit |
|---|---|
| Components |
|
-Supramolecule #1000: Ryanodine receptor 1 (calcium release channel) from rabbit
| Supramolecule | Name: Ryanodine receptor 1 (calcium release channel) from rabbit type: sample / ID: 1000 / Details: protein was reconstituted in lipid nanodiscs / Oligomeric state: tetramer / Number unique components: 1 |
|---|---|
| Molecular weight | Theoretical: 2.26 MDa |
-Macromolecule #1: Ryanodine receptor 1
| Macromolecule | Name: Ryanodine receptor 1 / type: protein_or_peptide / ID: 1 / Name.synonym: Skeletal muscle calcium release channel / Number of copies: 4 / Oligomeric state: tetramer / Recombinant expression: No |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 2.26 MDa |
| Sequence | UniProtKB: Ryanodine receptor 1 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 2 mg/mL |
|---|---|
| Buffer | pH: 7.4 Details: 10 mM MOPS, 200 mM NaCl, 10mM CaCl2, 2mM DTT, 0.2% fluorinated octyl-maltoside |
| Grid | Details: C-Flat CF-1.2/1.3-4C holey carbon grid |
| Vitrification | Cryogen name: ETHANE / Instrument: GATAN CRYOPLUNGE 3 Method: protein solution was applied on glow discharged grid and blotted for 1.5 seconds before plunging |
- Electron microscopy
Electron microscopy
| Microscope | JEOL 3200FSC |
|---|---|
| Temperature | Min: 80 K |
| Specialist optics | Energy filter - Name: in-column omega energy filter / Energy filter - Lower energy threshold: 0.0 eV / Energy filter - Upper energy threshold: 15.0 eV |
| Date | Mar 12, 2012 |
| Image recording | Category: CCD / Film or detector model: TVIPS TEMCAM-F816 (8k x 8k) / Number real images: 1041 / Average electron dose: 20 e/Å2 Details: Images were collected automatically with EM-TOOLS software |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 4.1 mm / Nominal defocus max: 0.0039 µm / Nominal defocus min: 0.0009 µm / Nominal magnification: 60000 |
| Sample stage | Specimen holder: liquid nitorgen cooled / Specimen holder model: JEOL 3200FSC CRYOHOLDER |
 Movie
Movie Controller
Controller














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)