[English] 日本語
 Yorodumi
Yorodumi- EMDB-27221: Cryo-EM map of human LIF signaling complex with full extracellula... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM map of human LIF signaling complex with full extracellular domains | |||||||||
 Map data Map data | LIF complex main map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | cytokine signaling / LIF / gp130 / LIFR / CYTOKINE | |||||||||
| Function / homology |  Function and homology information Function and homology informationleukemia inhibitory factor receptor binding / spongiotrophoblast differentiation / positive regulation of mesenchymal to epithelial transition involved in metanephros morphogenesis / meiotic nuclear division / leukemia inhibitory factor receptor activity / oncostatin-M-mediated signaling pathway / interleukin-27 receptor activity / muscle organ morphogenesis / ciliary neurotrophic factor receptor activity / negative regulation of meiotic nuclear division ...leukemia inhibitory factor receptor binding / spongiotrophoblast differentiation / positive regulation of mesenchymal to epithelial transition involved in metanephros morphogenesis / meiotic nuclear division / leukemia inhibitory factor receptor activity / oncostatin-M-mediated signaling pathway / interleukin-27 receptor activity / muscle organ morphogenesis / ciliary neurotrophic factor receptor activity / negative regulation of meiotic nuclear division / leukemia inhibitory factor signaling pathway / negative regulation of interleukin-6-mediated signaling pathway / oncostatin-M receptor complex / ciliary neurotrophic factor receptor binding / interleukin-11 receptor activity / interleukin-11 binding / RUNX1 regulates transcription of genes involved in interleukin signaling / ciliary neurotrophic factor-mediated signaling pathway / ciliary neurotrophic factor receptor complex / interleukin-27-mediated signaling pathway / interleukin-6 receptor complex / regulation of metanephric nephron tubule epithelial cell differentiation / lung lobe morphogenesis / positive regulation of peptidyl-serine phosphorylation of STAT protein / trophoblast giant cell differentiation / negative regulation of hormone secretion / cell surface receptor signaling pathway via STAT / lung vasculature development / interleukin-11-mediated signaling pathway / T-helper 17 cell lineage commitment / positive regulation of adaptive immune response / positive regulation of acute inflammatory response / positive regulation of macrophage differentiation / positive regulation of astrocyte differentiation / intestinal epithelial cell development / positive regulation of platelet aggregation / Interleukin-27 signaling / IL-6-type cytokine receptor ligand interactions / Interleukin-35 Signalling / positive regulation of cell adhesion mediated by integrin / cytokine receptor activity / lung alveolus development / glycogen metabolic process / Interleukin-6 signaling / interleukin-6-mediated signaling pathway / positive regulation of Notch signaling pathway / regulation of cell differentiation / protein tyrosine kinase activator activity / positive regulation of cardiac muscle hypertrophy / MAPK3 (ERK1) activation / cytokine binding / growth factor binding / Interleukin-10 signaling / MAPK1 (ERK2) activation / somatic stem cell population maintenance / macrophage differentiation / decidualization / positive regulation of vascular endothelial growth factor production / blood vessel remodeling / neuron development / positive regulation of osteoblast differentiation / coreceptor activity / positive regulation of T cell proliferation / positive regulation of tyrosine phosphorylation of STAT protein / embryo implantation / response to cytokine / cytokine activity / stem cell differentiation / growth factor activity / cell morphogenesis / negative regulation of ERK1 and ERK2 cascade / cytokine-mediated signaling pathway / positive regulation of peptidyl-tyrosine phosphorylation / positive regulation of fibroblast proliferation / positive regulation of peptidyl-serine phosphorylation / gene expression / scaffold protein binding / fibroblast proliferation / Interleukin-4 and Interleukin-13 signaling / negative regulation of neuron apoptotic process / positive regulation of MAPK cascade / receptor complex / cell surface receptor signaling pathway / response to hypoxia / immune response / membrane raft / negative regulation of cell population proliferation / external side of plasma membrane / signaling receptor binding / neuronal cell body / positive regulation of cell population proliferation / dendrite / positive regulation of gene expression / negative regulation of apoptotic process / positive regulation of transcription by RNA polymerase II / extracellular space / extracellular exosome / extracellular region / identical protein binding / membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.54 Å | |||||||||
 Authors Authors | Zhou Y / Franklin MC | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2023 Journal: Sci Adv / Year: 2023Title: Structural insights into the assembly of gp130 family cytokine signaling complexes. Authors: Yi Zhou / Panayiotis E Stevis / Jing Cao / Kei Saotome / Jiaxi Wu / Arielle Glatman Zaretsky / Sokol Haxhinasto / George D Yancopoulos / Andrew J Murphy / Mark W Sleeman / William C Olson / Matthew C Franklin /  Abstract: The interleukin-6 (IL-6) family cytokines signal through gp130 receptor homodimerization or heterodimerization with a second signaling receptor and play crucial roles in various cellular processes. ...The interleukin-6 (IL-6) family cytokines signal through gp130 receptor homodimerization or heterodimerization with a second signaling receptor and play crucial roles in various cellular processes. We determined cryo-electron microscopy structures of five signaling complexes of this family, containing full receptor ectodomains bound to their respective ligands ciliary neurotrophic factor, cardiotrophin-like cytokine factor 1 (CLCF1), leukemia inhibitory factor, IL-27, and IL-6. Our structures collectively reveal similarities and differences in the assembly of these complexes. The acute bends at both signaling receptors in all complexes bring the membrane-proximal domains to a ~30 angstrom range but with distinct distances and orientations. We also reveal how CLCF1 engages its secretion chaperone cytokine receptor-like factor 1. Our data provide valuable insights for therapeutically targeting gp130-mediated signaling. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_27221.map.gz emd_27221.map.gz | 229.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-27221-v30.xml emd-27221-v30.xml emd-27221.xml emd-27221.xml | 17.4 KB 17.4 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_27221.png emd_27221.png | 91.8 KB | ||
| Filedesc metadata |  emd-27221.cif.gz emd-27221.cif.gz | 6.6 KB | ||
| Others |  emd_27221_half_map_1.map.gz emd_27221_half_map_1.map.gz emd_27221_half_map_2.map.gz emd_27221_half_map_2.map.gz | 226.8 MB 226.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-27221 http://ftp.pdbj.org/pub/emdb/structures/EMD-27221 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27221 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27221 | HTTPS FTP |
-Validation report
| Summary document |  emd_27221_validation.pdf.gz emd_27221_validation.pdf.gz | 1.2 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_27221_full_validation.pdf.gz emd_27221_full_validation.pdf.gz | 1.2 MB | Display | |
| Data in XML |  emd_27221_validation.xml.gz emd_27221_validation.xml.gz | 15.8 KB | Display | |
| Data in CIF |  emd_27221_validation.cif.gz emd_27221_validation.cif.gz | 18.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27221 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27221 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27221 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27221 | HTTPS FTP |
-Related structure data
| Related structure data |  8d6aMC  8d74C  8d7eC  8d7hC  8d7rC  8d82C  8d85C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_27221.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_27221.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | LIF complex main map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.86 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: half map A
| File | emd_27221_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
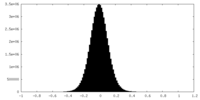
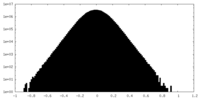
| Density Histograms |
-Half map: half map B
| File | emd_27221_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
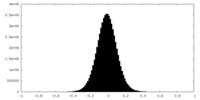
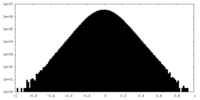
| Density Histograms |
- Sample components
Sample components
-Entire : Human LIF in complex with gp130 and LIFR
| Entire | Name: Human LIF in complex with gp130 and LIFR |
|---|---|
| Components |
|
-Supramolecule #1: Human LIF in complex with gp130 and LIFR
| Supramolecule | Name: Human LIF in complex with gp130 and LIFR / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Leukemia inhibitory factor
| Macromolecule | Name: Leukemia inhibitory factor / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 19.652777 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: PLPITPVNAT CAIRHPCHNN LMNQIRSQLA QLNGSANALF ILYYTAQGEP FPNNLDKLCG PNVTDFPPFH ANGTEKAKLV ELYRIVVYL GTSLGNITRD QKILNPSALS LHSKLNATAD ILRGLLSNVL CRLCSKYHVG HVDVTYGPDT SGKDVFQKKK L GCQLLGKY KQIIAVLAQA F UniProtKB: Leukemia inhibitory factor |
-Macromolecule #2: Interleukin-6 receptor subunit beta
| Macromolecule | Name: Interleukin-6 receptor subunit beta / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 71.233203 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: ELLDPCGYIS PESPVVQLHS NFTAVCVLKE KCMDYFHVNA NYIVWKTNHF TIPKEQYTII NRTASSVTFT DIASLNIQLT CNILTFGQL EQNVYGITII SGLPPEKPKN LSCIVNEGKK MRCEWDGGRE THLETNFTLK SEWATHKFAD CKAKRDTPTS C TVDYSTVY ...String: ELLDPCGYIS PESPVVQLHS NFTAVCVLKE KCMDYFHVNA NYIVWKTNHF TIPKEQYTII NRTASSVTFT DIASLNIQLT CNILTFGQL EQNVYGITII SGLPPEKPKN LSCIVNEGKK MRCEWDGGRE THLETNFTLK SEWATHKFAD CKAKRDTPTS C TVDYSTVY FVNIEVWVEA ENALGKVTSD HINFDPVYKV KPNPPHNLSV INSEELSSIL KLTWTNPSIK SVIILKYNIQ YR TKDASTW SQIPPEDTAS TRSSFTVQDL KPFTEYVFRI RCMKEDGKGY WSDWSEEASG ITYEDRPSKA PSFWYKIDPS HTQ GYRTVQ LVWKTLPPFE ANGKILDYEV TLTRWKSHLQ NYTVNATKLT VNLTNDRYLA TLTVRNLVGK SDAAVLTIPA CDFQ ATHPV MDLKAFPKDN MLWVEWTTPR ESVKKYILEW CVLSDKAPCI TDWQQEDGTV HRTYLRGNLA ESKCYLITVT PVYAD GPGS PESIKAYLKQ APPSKGPTVR TKKVGKNEAV LEWDQLPVDV QNGFIRNYTI FYRTIIGNET AVNVDSSHTE YTLSSL TSD TLYMVRMAAY TDEGGKDGPE FTFTTPKFAQ GEIEEQKLIS EEDLGGEQKL ISEEDLHHHH HH UniProtKB: Interleukin-6 receptor subunit beta |
-Macromolecule #3: Leukemia inhibitory factor receptor
| Macromolecule | Name: Leukemia inhibitory factor receptor / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 92.834812 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: QKKGAPHDLK CVTNNLQVWN CSWKAPSGTG RGTDYEVCIE NRSRSCYQLE KTSIKIPALS HGDYEITINS LHDFGSSTSK FTLNEQNVS LIPDTPEILN LSADFSTSTL YLKWNDRGSV FPHRSNVIWE IKVLRKESME LVKLVTHNTT LNGKDTLHHW S WASDMPLE ...String: QKKGAPHDLK CVTNNLQVWN CSWKAPSGTG RGTDYEVCIE NRSRSCYQLE KTSIKIPALS HGDYEITINS LHDFGSSTSK FTLNEQNVS LIPDTPEILN LSADFSTSTL YLKWNDRGSV FPHRSNVIWE IKVLRKESME LVKLVTHNTT LNGKDTLHHW S WASDMPLE CAIHFVEIRC YIDNLHFSGL EEWSDWSPVK NISWIPDSQT KVFPQDKVIL VGSDITFCCV SQEKVLSALI GH TNCPLIH LDGENVAIKI RNISVSASSG TNVVFTTEDN IFGTVIFAGY PPDTPQQLNC ETHDLKEIIC SWNPGRVTAL VGP RATSYT LVESFSGKYV RLKRAEAPTN ESYQLLFQML PNQEIYNFTL NAHNPLGRSQ STILVNITEK VYPHTPTSFK VKDI NSTAV KLSWHLPGNF AKINFLCEIE IKKSNSVQEQ RNVTIKGVEN SSYLVALDKL NPYTLYTFRI RCSTETFWKW SKWSN KKQH LTTEASPSKG PDTWREWSSD GKNLIIYWKP LPINEANGKI LSYNVSCSSD EETQSLSEIP DPQHKAEIRL DKNDYI ISV VAKNSVGSSP PSKIASMEIP NDDLKIEQVV GMGKGILLTW HYDPNMTCDY VIKWCNSSRS EPCLMDWRKV PSNSTET VI ESDEFRPGIR YNFFLYGCRN QGYQLLRSMI GYIEELAPIV APNFTVEDTS ADSILVKWED IPVEELRGFL RGYLFYFG K GERDTSKMRV LESGRSDIKV KNITDISQKT LRIADLQGKT SYHLVLRAYT DGGVGPEKSM YVVTKENSEQ KLISEEDLG GEQKLISEED LHHHHHH UniProtKB: Leukemia inhibitory factor receptor |
-Macromolecule #5: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 5 / Number of copies: 5 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.6 µm / Nominal defocus min: 1.4000000000000001 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Resolution.type: BY AUTHOR / Resolution: 3.54 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 171328 |
| Initial angle assignment | Type: RANDOM ASSIGNMENT |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller





















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)