[English] 日本語
 Yorodumi
Yorodumi- EMDB-26192: Cryo-EM structure of the basal state of the Artemis:DNA-PKcs comp... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the basal state of the Artemis:DNA-PKcs complex (see COMPND 13/14) | |||||||||
 Map data Map data | 3.33A (FSC = 0.143) | |||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationMHC class II antigen presentation / nonhomologous end joining complex / single-stranded DNA endodeoxyribonuclease activity / Neutrophil degranulation / V(D)J recombination / entry into host cell by a symbiont-containing vacuole / 5'-3' exonuclease activity / 5'-3' DNA exonuclease activity / response to ionizing radiation / Hydrolases; Acting on peptide bonds (peptidases); Aspartic endopeptidases ...MHC class II antigen presentation / nonhomologous end joining complex / single-stranded DNA endodeoxyribonuclease activity / Neutrophil degranulation / V(D)J recombination / entry into host cell by a symbiont-containing vacuole / 5'-3' exonuclease activity / 5'-3' DNA exonuclease activity / response to ionizing radiation / Hydrolases; Acting on peptide bonds (peptidases); Aspartic endopeptidases / protein autoprocessing / interstrand cross-link repair / transport vesicle / telomere maintenance / B cell differentiation / Nonhomologous End-Joining (NHEJ) / protein processing / double-strand break repair via nonhomologous end joining / endonuclease activity / adaptive immune response / damaged DNA binding / Hydrolases; Acting on ester bonds / aspartic-type endopeptidase activity / Golgi apparatus / proteolysis / nucleoplasm Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  human (human) human (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.33 Å | |||||||||
 Authors Authors | Watanabe G / Lieber MR / Williams DR | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Nucleic Acids Res / Year: 2022 Journal: Nucleic Acids Res / Year: 2022Title: Structural analysis of the basal state of the Artemis:DNA-PKcs complex. Authors: Go Watanabe / Michael R Lieber / Dewight R Williams /  Abstract: Artemis nuclease and DNA-dependent protein kinase catalytic subunit (DNA-PKcs) are key components in nonhomologous DNA end joining (NHEJ), the major repair mechanism for double-strand DNA breaks. ...Artemis nuclease and DNA-dependent protein kinase catalytic subunit (DNA-PKcs) are key components in nonhomologous DNA end joining (NHEJ), the major repair mechanism for double-strand DNA breaks. Artemis activation by DNA-PKcs resolves hairpin DNA ends formed during V(D)J recombination. Artemis deficiency disrupts development of adaptive immunity and leads to radiosensitive T- B- severe combined immunodeficiency (RS-SCID). An activated state of Artemis in complex with DNA-PK was solved by cryo-EM recently, which showed Artemis bound to the DNA. Here, we report that the pre-activated form (basal state) of the Artemis:DNA-PKcs complex is stable on an agarose-acrylamide gel system, and suitable for cryo-EM structural analysis. Structures show that the Artemis catalytic domain is dynamically positioned externally to DNA-PKcs prior to ABCDE autophosphorylation and show how both the catalytic and regulatory domains of Artemis interact with the N-HEAT and FAT domains of DNA-PKcs. We define a mutually exclusive binding site for Artemis and XRCC4 on DNA-PKcs and show that an XRCC4 peptide disrupts the Artemis:DNA-PKcs complex. All of the findings are useful in explaining how a hypomorphic L3062R missense mutation of DNA-PKcs could lead to insufficient Artemis activation, hence RS-SCID. Our results provide various target site candidates to design disruptors for Artemis:DNA-PKcs complex formation. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_26192.map.gz emd_26192.map.gz | 483.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-26192-v30.xml emd-26192-v30.xml emd-26192.xml emd-26192.xml | 27.3 KB 27.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_26192_fsc.xml emd_26192_fsc.xml | 17.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_26192.png emd_26192.png | 120.9 KB | ||
| Masks |  emd_26192_msk_1.map emd_26192_msk_1.map | 512 MB |  Mask map Mask map | |
| Others |  emd_26192_additional_1.map.gz emd_26192_additional_1.map.gz emd_26192_additional_2.map.gz emd_26192_additional_2.map.gz emd_26192_half_map_1.map.gz emd_26192_half_map_1.map.gz emd_26192_half_map_2.map.gz emd_26192_half_map_2.map.gz | 262.7 MB 7.6 MB 475.4 MB 475.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-26192 http://ftp.pdbj.org/pub/emdb/structures/EMD-26192 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26192 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26192 | HTTPS FTP |
-Validation report
| Summary document |  emd_26192_validation.pdf.gz emd_26192_validation.pdf.gz | 980.9 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_26192_full_validation.pdf.gz emd_26192_full_validation.pdf.gz | 980.4 KB | Display | |
| Data in XML |  emd_26192_validation.xml.gz emd_26192_validation.xml.gz | 26.5 KB | Display | |
| Data in CIF |  emd_26192_validation.cif.gz emd_26192_validation.cif.gz | 34.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26192 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26192 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26192 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26192 | HTTPS FTP |
-Related structure data
| Related structure data |  7tyrMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_26192.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_26192.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | 3.33A (FSC = 0.143) | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.08 Å | ||||||||||||||||||||||||||||||||||||
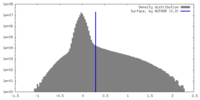
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_26192_msk_1.map emd_26192_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
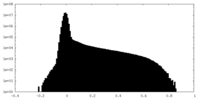
| Density Histograms |
-Additional map: Blurred map
| File | emd_26192_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Blurred map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Density modified map: 3.18A (FSC = 0.5)
| File | emd_26192_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Density modified map: 3.18A (FSC = 0.5) | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half Map 1
| File | emd_26192_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half Map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half Map 2
| File | emd_26192_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half Map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : A complex of Artemis:DNA-PKcs
| Entire | Name: A complex of Artemis:DNA-PKcs |
|---|---|
| Components |
|
-Supramolecule #1: A complex of Artemis:DNA-PKcs
| Supramolecule | Name: A complex of Artemis:DNA-PKcs / type: complex / Chimera: Yes / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: DNA-dependent protein kinase catalytic subunit
| Macromolecule | Name: DNA-dependent protein kinase catalytic subunit / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO / EC number: non-specific serine/threonine protein kinase |
|---|---|
| Source (natural) | Organism:  human (human) human (human) |
| Molecular weight | Theoretical: 469.673219 KDa |
| Sequence | String: MAGSGAGVRC SLLRLQETLS AADRCGAALA GHQLIRGLGQ ECVLSSSPAV LALQTSLVFS RDFGLLVFVR KSLNSIEFRE CREEILKFL CIFLEKMGQK IAPYSVEIKN TCTSVYTKDR AAKCKIPALD LLIKLLQTFR SSRLMDEFKI GELFSKFYGE L ALKKKIPD ...String: MAGSGAGVRC SLLRLQETLS AADRCGAALA GHQLIRGLGQ ECVLSSSPAV LALQTSLVFS RDFGLLVFVR KSLNSIEFRE CREEILKFL CIFLEKMGQK IAPYSVEIKN TCTSVYTKDR AAKCKIPALD LLIKLLQTFR SSRLMDEFKI GELFSKFYGE L ALKKKIPD TVLEKVYELL GLLGEVHPSE MINNAENLFR AFLGELKTQM TSAVREPKLP VLAGCLKGLS SLLCNFTKSM EE DPQTSRE IFNFVLKAIR PQIDLKRYAV PSAGLRLFAL HASQFSTCLL DNYVSLFEVL LKWCAHTNVE LKKAALSALE SFL KQVSNM VAKNAEMHKN KLQYFMEQFY GIIRNVDSNN KELSIAIRGY GLFAGPCKVI NAKDVDFMYV ELIQRCKQMF LTQT DTGDD RVYQMPSFLQ SVASVLLYLD TVPEVYTPVL EHLVVMQIDS FPQYSPKMQL VCCRAIVKVF LALAAKGPVL RNCIS TVVH QGLIRICSKP VVLPKGPESE SEDHRASGEV RTGKWKVPTY KDYVDLFRHL LSSDQMMDSI LADEAFFSVN SSSESL NHL LYDEFVKSVL KIVEKLDLTL EIQTVGEQEN GDEAPGVWMI PTSDPAANLH PAKPKDFSAF INLVEFCREI LPEKQAE FF EPWVYSFSYE LILQSTRLPL ISGFYKLLSI TVRNAKKIKY FEGVSPKSLK HSPEDPEKYS CFALFVKFGK EVAVKMKQ Y KDELLASCLT FLLSLPHNII ELDVRAYVPA LQMAFKLGLS YTPLAEVGLN ALEEWSIYID RHVMQPYYKD ILPCLDGYL KTSALSDETK NNWEVSALSR AAQKGFNKVV LKHLKKTKNL SSNEAISLEE IRIRVVQMLG SLGGQINKNL LTVTSSDEMM KSYVAWDRE KRLSFAVPFR EMKPVIFLDV FLPRVTELAL TASDRQTKVA ACELLHSMVM FMLGKATQMP EGGQGAPPMY Q LYKRTFPV LLRLACDVDQ VTRQLYEPLV MQLIHWFTNN KKFESQDTVA LLEAILDGIV DPVDSTLRDF CGRCIREFLK WS IKQITPQ QQEKSPVNTK SLFKRLYSLA LHPNAFKRLG ASLAFNNIYR EFREEESLVE QFVFEALVIY MESLALAHAD EKS LGTIQQ CCDAIDHLCR IIEKKHVSLN KAKKRRLPRG FPPSASLCLL DLVKWLLAHC GRPQTECRHK SIELFYKFVP LLPG NRSPN LWLKDVLKEE GVSFLINTFE GGGCGQPSGI LAQPTLLYLR GPFSLQATLC WLDLLLAALE CYNTFIGERT VGALQ VLGT EAQSSLLKAV AFFLESIAMH DIIAAEKCFG TGAAGNRTSP QEGERYNYSK CTVVVRIMEF TTTLLNTSPE GWKLLK KDL CNTHLMRVLV QTLCEPASIG FNIGDVQVMA HLPDVCVNLM KALKMSPYKD ILETHLREKI TAQSIEELCA VNLYGPD AQ VDRSRLAAVV SACKQLHRAG LLHNILPSQS TDLHHSVGTE LLSLVYKGIA PGDERQCLPS LDLSCKQLAS GLLELAFA F GGLCERLVSL LLNPAVLSTA SLGSSQGSVI HFSHGEYFYS LFSETINTEL LKNLDLAVLE LMQSSVDNTK MVSAVLNGM LDQSFRERAN QKHQGLKLAT TILQHWKKCD SWWAKDSPLE TKMAVLALLA KILQIDSSVS FNTSHGSFPE VFTTYISLLA DTKLDLHLK GQAVTLLPFF TSLTGGSLEE LRRVLEQLIV AHFPMQSREF PPGTPRFNNY VDCMKKFLDA LELSQSPMLL E LMTEVLCR EQQHVMEELF QSSFRRIARR GSCVTQVGLL ESVYEMFRKD DPRLSFTRQS FVDRSLLTLL WHCSLDALRE FF STIVVDA IDVLKSRFTK LNESTFDTQI TKKMGYYKIL DVMYSRLPKD DVHAKESKIN QVFHGSCITE GNELTKTLIK LCY DAFTEN MAGENQLLER RRLYHCAAYN CAISVICCVF NELKFYQGFL FSEKPEKNLL IFENLIDLKR RYNFPVEVEV PMER KKKYI EIRKEAREAA NGDSDGPSYM SSLSYLADST LSEEMSQFDF STGVQSYSYS SQDPRPATGR FRRREQRDPT VHDDV LELE MDELNRHECM APLTALVKHM HRSLGPPQGE EDSVPRDLPS WMKFLHGKLG NPIVPLNIRL FLAKLVINTE EVFRPY AKH WLSPLLQLAA SENNGGEGIH YMVVEIVATI LSWTGLATPT GVPKDEVLAN RLLNFLMKHV FHPKRAVFRH NLEIIKT LV ECWKDCLSIP YRLIFEKFSG KDPNSKDNSV GIQLLGIVMA NDLPPYDPQC GIQSSEYFQA LVNNMSFVRY KEVYAAAA E VLGLILRYVM ERKNILEESL CELVAKQLKQ HQNTMEDKFI VCLNKVTKSF PPLADRFMNA VFFLLPKFHG VLKTLCLEV VLCRVEGMTE LYFQLKSKDF VQVMRHRDDE RQKVCLDIIY KMMPKLKPVE LRELLNPVVE FVSHPSTTCR EQMYNILMWI HDNYRDPES ETDNDSQEIF KLAKDVLIQG LIDENPGLQL IIRNFWSHET RLPSNTLDRL LALNSLYSPK IEVHFLSLAT N FLLEMTSM SPDYPNPMFE HPLSECEFQE YTIDSDWRFR STVLTPMFVE TQASQGTLQT RTQEGSLSAR WPVAGQIRAT QQ QHDFTLT QTADGRSSFD WLTGSSTDPL VDHTSPSSDS LLFAHKRSER LQRAPLKSVG PDFGKKRLGL PGDEVDNKVK GAA GRTDLL RLRRRFMRDQ EKLSLMYARK GVAEQKREKE IKSELKMKQD AQVVLYRSYR HGDLPDIQIK HSSLITPLQA VAQR DPIIA KQLFSSLFSG ILKEMDKFKT LSEKNNITQK LLQDFNRFLN TTFSFFPPFV SCIQDISCQH AALLSLDPAA VSAGC LASL QQPVGIRLLE EALLRLLPAE LPAKRVRGKA RLPPDVLRWV ELAKLYRSIG EYDVLRGIFT SEIGTKQITQ SALLAE ARS DYSEAAKQYD EALNKQDWVD GEPTEAEKDF WELASLDCYN HLAEWKSLEY CSTASIDSEN PPDLNKIWSE PFYQETY LP YMIRSKLKLL LQGEADQSLL TFIDKAMHGE LQKAILELHY SQELSLLYLL QDDVDRAKYY IQNGIQSFMQ NYSSIDVL L HQSRLTKLQS VQALTEIQEF ISFISKQGNL SSQVPLKRLL NTWTNRYPDA KMDPMNIWDD IITNRCFFLS KIEEKLTPL PEDNSMNVDQ DGDPSDRMEV QEQEEDISSL IRSCKFSMKM KMIDSARKQN NFSLAMKLLK ELHKESKTRD DWLVSWVQSY CRLSHCRSR SQGCSEQVLT VLKTVSLLDE NNVSSYLSKN ILAFRDQNIL LGTTYRIIAN ALSSEPACLA EIEEDKARRI L ELSGSSSE DSEKVIAGLY QRAFQHLSEA VQAAEEEAQP PSWSCGPAAG VIDAYMTLAD FCDQQLRKEE ENASVIDSAE LQ AYPALVV EKMLKALKLN SNEARLKFPR LLQIIERYPE ETLSLMTKEI SSVPCWQFIS WISHMVALLD KDQAVAVQHS VEE ITDNYP QAIVYPFIIS SESYSFKDTS TGHKNKEFVA RIKSKLDQGG VIQDFINALD QLSNPELLFK DWSNDVRAEL AKTP VNKKN IEKMYERMYA ALGDPKAPGL GAFRRKFIQT FGKEFDKHFG KGGSKLLRMK LSDFNDITNM LLLKMNKDSK PPGNL KECS PWMSDFKVEF LRNELEIPGQ YDGRGKPLPE YHVRIAGFDE RVTVMASLRR PKRIIIRGHD EREHPFLVKG GEDLRQ DQR VEQLFQVMNG ILAQDSACSQ RALQLRTYSV VPMTSRLGLI EWLENTVTLK DLLLNTMSQE EKAAYLSDPR APPCEYK DW LTKMSGKHDV GAYMLMYKGA NRTETVTSFR KRESKVPADL LKRAFVRMST SPEAFLALRS HFASSHALIC ISHWILGI G DRHLNNFMVA METGGVIGID FGHAFGSATQ FLPVPELMPF RLTRQFINLM LPMKETGLMY SIMVHALRAF RSDPGLLTN TMDVFVKEPS FDWKNFEQKM LKKGGSWIQE INVAEKNWYP RQKICYAKRK LAGANPAVIT CDELLLGHEK APAFRDYVAV ARGSKDHNI RAQEPESGLS EETQVKCLMD QATDPNILGR TWEGWEPWM |
-Macromolecule #2: Protein artemis
| Macromolecule | Name: Protein artemis / type: protein_or_peptide / ID: 2 Details: Please use a 'blurred' map of the EMD-26192 entry to see the density of the Artemis catalytic region Number of copies: 1 / Enantiomer: LEVO / EC number: Hydrolases; Acting on ester bonds |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 80.493352 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MSSFEGQMAE YPTISIDRFD RENLRARAYF LSHCHKDHMK GLRAPTLKRR LECSLKVYLY CSPVTKELLL TSPKYRFWKK RIISIEIET PTQISLVDEA SGEKEEIVVT LLPAGHCPGS VMFLFQGNNG TVLYTGDFRL AQGEAARMEL LHSGGRVKDI Q SVYLDTTF ...String: MSSFEGQMAE YPTISIDRFD RENLRARAYF LSHCHKDHMK GLRAPTLKRR LECSLKVYLY CSPVTKELLL TSPKYRFWKK RIISIEIET PTQISLVDEA SGEKEEIVVT LLPAGHCPGS VMFLFQGNNG TVLYTGDFRL AQGEAARMEL LHSGGRVKDI Q SVYLDTTF CDPRFYQIPS REECLSGVLE LVRSWITRSP YHVVWLNCKA AYGYEYLFTN LSEELGVQVH VNKLDMFRNM PE ILHHLTT DRNTQIHACR HPKAEEYFQW SKLPCGITSR NRIPLHIISI KPSTMWFGER SRKTNVIVRT GESSYRACFS FHS SYSEIK DFLSYLCPVN AYPNVIPVGT TMDKVVEILK PLCRSSQSTE PKYKPLGKLK RARTVHRDSE EEDDYLFDDP LPIP LRHKV PYPETFHPEV FSMTAVSEKQ PEKLRQTPGC CRAECMQSSR FTNFVDCEES NSESEEEVGI PASLQGDLGS VLHLQ KADG DVPQWEVFFK RNDEITDESL ENFPSSTVAG GSQSPKLFSD SDGESTHISS QNSSQSTHIT EQGSQGWDSQ SDTVLL SSQ ERNSGDITSL DKADYRPTIK ENIPASLMEQ NVICPKDTYS DLKSRDKDVT IVPSTGEPTT LSSETHIPEE KSLLNLS TN ADSQSSSDFE VPSTPEAELP KREHLQYLYE KLATGESIAV KKRKCSLLDT ENLYFQGHHH HHHHH |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 Component:
| |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grid | Model: UltrAuFoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 0.03 kPa | |||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 73 % / Chamber temperature: 295 K Details: Plunge-freeze was performed using a home-made manual plunger at typical indoor humidity (Los Angeles, CA) and at room temperature.. | |||||||||||||||
| Details | This sample was monodisperse. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Digitization - Dimensions - Width: 5760 pixel / Digitization - Dimensions - Height: 4092 pixel / Average exposure time: 3.8 sec. / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Calibrated magnification: 46296 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 3.0 µm / Nominal defocus min: 0.75 µm / Nominal magnification: 81000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)