[English] 日本語
 Yorodumi
Yorodumi- EMDB-24439: HUMAN IMPDH1 TREATED WITH GTP, IMP, AND NAD+; INTERFACE-CENTERED -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-24439 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | HUMAN IMPDH1 TREATED WITH GTP, IMP, AND NAD+; INTERFACE-CENTERED | ||||||||||||
 Map data Map data | HUMAN IMPDH1 TREATED WITH GTP, IMP, AND NAD+ INTERFACE-CENTERED | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | METABOLISM / FILAMENT / ALLOSTERY / ADENINE / OXIDOREDUCTASE | ||||||||||||
| Function / homology |  Function and homology information Function and homology information'de novo' XMP biosynthetic process / Purine ribonucleoside monophosphate biosynthesis / lymphocyte proliferation / IMP dehydrogenase / IMP dehydrogenase activity / GMP biosynthetic process / Azathioprine ADME / GTP biosynthetic process / azurophil granule lumen / secretory granule lumen ...'de novo' XMP biosynthetic process / Purine ribonucleoside monophosphate biosynthesis / lymphocyte proliferation / IMP dehydrogenase / IMP dehydrogenase activity / GMP biosynthetic process / Azathioprine ADME / GTP biosynthetic process / azurophil granule lumen / secretory granule lumen / ficolin-1-rich granule lumen / Potential therapeutics for SARS / nucleic acid binding / nucleotide binding / Neutrophil degranulation / DNA binding / RNA binding / extracellular region / metal ion binding / identical protein binding / nucleus / cytoplasm / cytosol Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.6 Å | ||||||||||||
 Authors Authors | Burrell AL / Kollman JM | ||||||||||||
| Funding support |  United States, 3 items United States, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2022 Journal: Nat Struct Mol Biol / Year: 2022Title: IMPDH1 retinal variants control filament architecture to tune allosteric regulation. Authors: Anika L Burrell / Chuankai Nie / Meerit Said / Jacqueline C Simonet / David Fernández-Justel / Matthew C Johnson / Joel Quispe / Rubén M Buey / Jeffrey R Peterson / Justin M Kollman /   Abstract: Inosine-5'-monophosphate dehydrogenase (IMPDH), a key regulatory enzyme in purine nucleotide biosynthesis, dynamically assembles filaments in response to changes in metabolic demand. Humans have two ...Inosine-5'-monophosphate dehydrogenase (IMPDH), a key regulatory enzyme in purine nucleotide biosynthesis, dynamically assembles filaments in response to changes in metabolic demand. Humans have two isoforms: IMPDH2 filaments reduce sensitivity to feedback inhibition, while IMPDH1 assembly remains uncharacterized. IMPDH1 plays a unique role in retinal metabolism, and point mutants cause blindness. Here, in a series of cryogenic-electron microscopy structures we show that human IMPDH1 assembles polymorphic filaments with different assembly interfaces in extended and compressed states. Retina-specific splice variants introduce structural elements that reduce sensitivity to GTP inhibition, including stabilization of the extended filament form. Finally, we show that IMPDH1 disease mutations fall into two classes: one disrupts GTP regulation and the other has no effect on GTP regulation or filament assembly. These findings provide a foundation for understanding the role of IMPDH1 in retinal function and disease and demonstrate the diverse mechanisms by which metabolic enzyme filaments are allosterically regulated. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_24439.map.gz emd_24439.map.gz | 115.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-24439-v30.xml emd-24439-v30.xml emd-24439.xml emd-24439.xml | 11.3 KB 11.3 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_24439.png emd_24439.png | 134.4 KB | ||
| Filedesc metadata |  emd-24439.cif.gz emd-24439.cif.gz | 5.5 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-24439 http://ftp.pdbj.org/pub/emdb/structures/EMD-24439 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24439 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24439 | HTTPS FTP |
-Related structure data
| Related structure data |  7rfeMC  7rerC  7resC  7rffC  7rfgC  7rfhC  7rfiC  7rgdC  7rgiC  7rglC  7rgmC  7rgqC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_24439.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_24439.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | HUMAN IMPDH1 TREATED WITH GTP, IMP, AND NAD+ INTERFACE-CENTERED | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.05 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Assembly interface of IMPDH1 filament bound to ATP, IMP, NAD+
| Entire | Name: Assembly interface of IMPDH1 filament bound to ATP, IMP, NAD+ |
|---|---|
| Components |
|
-Supramolecule #1: Assembly interface of IMPDH1 filament bound to ATP, IMP, NAD+
| Supramolecule | Name: Assembly interface of IMPDH1 filament bound to ATP, IMP, NAD+ type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 55405 MDa |
-Macromolecule #1: Inosine-5'-monophosphate dehydrogenase 1
| Macromolecule | Name: Inosine-5'-monophosphate dehydrogenase 1 / type: protein_or_peptide / ID: 1 / Number of copies: 8 / Enantiomer: LEVO / EC number: IMP dehydrogenase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 54.461527 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GYVPEDGLTA QQLFASADGL TYNDFLILPG FIDFIADEVD LTSALTRKIT LKTPLISSPM DTVTEADMAI AMALMGGIGF IHHNCTPEF QANEVRKVKK FEQGFITDPV VLSPSHTVGD VLEAKMRHGF SGIPITETGT MGSKLVGIVT SRDIDFLAEK D HTTLLSEV ...String: GYVPEDGLTA QQLFASADGL TYNDFLILPG FIDFIADEVD LTSALTRKIT LKTPLISSPM DTVTEADMAI AMALMGGIGF IHHNCTPEF QANEVRKVKK FEQGFITDPV VLSPSHTVGD VLEAKMRHGF SGIPITETGT MGSKLVGIVT SRDIDFLAEK D HTTLLSEV MTPRIELVVA PAGVTLKEAN EILQRSKKGK LPIVNDCDEL VAIIARTDLK KNRDYPLASK DSQKQLLCGA AV GTREDDK YRLDLLTQAG VDVIVLDSSQ GNSVYQIAMV HYIKQKYPHL QVIGGNVVTA AQAKNLIDAG VDGLRVGMGC GSI CITQEV MACGRPQGTA VYKVAEYARR FGVPIIADGG IQTVGHVVKA LALGASTVMM GSLLAATTEA PGEYFFSDGV RLKK YRGMG SLDAMEKSSS SQKRYFSEGD KVKIAQGVSG SIQDKGSIQK FVPYLIAGIQ HGCQDIGARS LSVLRSMMYS GELKF EKRT MSAQIEGGVH GLHSYEKRLY UniProtKB: Inosine-5'-monophosphate dehydrogenase 1 |
-Macromolecule #2: GUANOSINE-5'-TRIPHOSPHATE
| Macromolecule | Name: GUANOSINE-5'-TRIPHOSPHATE / type: ligand / ID: 2 / Number of copies: 16 / Formula: GTP |
|---|---|
| Molecular weight | Theoretical: 523.18 Da |
| Chemical component information |  ChemComp-GTP: |
-Macromolecule #3: ADENOSINE-5'-TRIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-TRIPHOSPHATE / type: ligand / ID: 3 / Number of copies: 8 / Formula: ATP |
|---|---|
| Molecular weight | Theoretical: 507.181 Da |
| Chemical component information |  ChemComp-ATP: |
-Macromolecule #4: INOSINIC ACID
| Macromolecule | Name: INOSINIC ACID / type: ligand / ID: 4 / Number of copies: 8 / Formula: IMP |
|---|---|
| Molecular weight | Theoretical: 348.206 Da |
| Chemical component information | 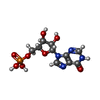 ChemComp-I: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Buffer | pH: 7 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: EMDB MAP |
|---|---|
| Final reconstruction | Applied symmetry - Point group: D4 (2x4 fold dihedral) / Resolution.type: BY AUTHOR / Resolution: 2.6 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 42500 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller





















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





















