[English] 日本語
 Yorodumi
Yorodumi- EMDB-2414: Signaling in Chemoreceptor Arrays Through Mobility Control of Kin... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-2414 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Signaling in Chemoreceptor Arrays Through Mobility Control of Kinase Domains - kinase activity state locked off - tsr mutant M222R | |||||||||
 Map data Map data | subvolume average of receptor array of tsr variant M222R | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | chemotaxis / E.coli / CheA | |||||||||
| Biological species |  | |||||||||
| Method | subtomogram averaging / cryo EM / Resolution: 30.0 Å | |||||||||
 Authors Authors | Briegel A / Ames P / Gumbart JC / Oikonomou CM / Parkinson JS / Jensen GJ | |||||||||
 Citation Citation |  Journal: Mol Microbiol / Year: 2013 Journal: Mol Microbiol / Year: 2013Title: The mobility of two kinase domains in the Escherichia coli chemoreceptor array varies with signalling state. Authors: Ariane Briegel / Peter Ames / James C Gumbart / Catherine M Oikonomou / John S Parkinson / Grant J Jensen /  Abstract: Motile bacteria sense their physical and chemical environment through highly cooperative, ordered arrays of chemoreceptors. These signalling complexes phosphorylate a response regulator which in turn ...Motile bacteria sense their physical and chemical environment through highly cooperative, ordered arrays of chemoreceptors. These signalling complexes phosphorylate a response regulator which in turn governs flagellar motor reversals, driving cells towards favourable environments. The structural changes that translate chemoeffector binding into the appropriate kinase output are not known. Here, we apply high-resolution electron cryotomography to visualize mutant chemoreceptor signalling arrays in well-defined kinase activity states. The arrays were well ordered in all signalling states, with no discernible differences in receptor conformation at 2-3 nm resolution. Differences were observed, however, in a keel-like density that we identify here as CheA kinase domains P1 and P2, the phosphorylation site domain and the binding domain for response regulator target proteins. Mutant receptor arrays with high kinase activities all exhibited small keels and high proteolysis susceptibility, indicative of mobile P1 and P2 domains. In contrast, arrays in kinase-off signalling states exhibited a range of keel sizes. These findings confirm that chemoreceptor arrays do not undergo large structural changes during signalling, and suggest instead that kinase activity is modulated at least in part by changes in the mobility of key domains. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_2414.map.gz emd_2414.map.gz | 698.4 KB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-2414-v30.xml emd-2414-v30.xml emd-2414.xml emd-2414.xml | 9.2 KB 9.2 KB | Display Display |  EMDB header EMDB header |
| Images |  M222R.tif M222R.tif | 248.7 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-2414 http://ftp.pdbj.org/pub/emdb/structures/EMD-2414 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2414 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2414 | HTTPS FTP |
-Validation report
| Summary document |  emd_2414_validation.pdf.gz emd_2414_validation.pdf.gz | 256.2 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_2414_full_validation.pdf.gz emd_2414_full_validation.pdf.gz | 255.4 KB | Display | |
| Data in XML |  emd_2414_validation.xml.gz emd_2414_validation.xml.gz | 5.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-2414 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-2414 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-2414 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-2414 | HTTPS FTP |
-Related structure data
| Related structure data |  5541C  5542C  5543C  5545C  5546C  5547C  5548C  5549C  5550C  5716C C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_2414.map.gz / Format: CCP4 / Size: 825.2 KB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_2414.map.gz / Format: CCP4 / Size: 825.2 KB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | subvolume average of receptor array of tsr variant M222R | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 6.44 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
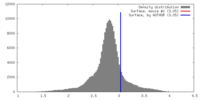
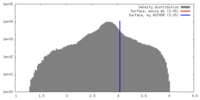
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : chemoreceptor array with receptor variant tsr M222R
| Entire | Name: chemoreceptor array with receptor variant tsr M222R |
|---|---|
| Components |
|
-Supramolecule #1000: chemoreceptor array with receptor variant tsr M222R
| Supramolecule | Name: chemoreceptor array with receptor variant tsr M222R / type: sample / ID: 1000 / Number unique components: 3 |
|---|
-Macromolecule #1: tsr M222R
| Macromolecule | Name: tsr M222R / type: protein_or_peptide / ID: 1 / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:  |
| Recombinant expression | Organism:  |
-Macromolecule #2: CheW
| Macromolecule | Name: CheW / type: protein_or_peptide / ID: 2 / Recombinant expression: No |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #3: CheA
| Macromolecule | Name: CheA / type: protein_or_peptide / ID: 3 / Oligomeric state: dimer / Recombinant expression: No |
|---|---|
| Source (natural) | Organism:  |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | subtomogram averaging |
| Aggregation state | cell |
- Sample preparation
Sample preparation
| Buffer | Details: Tryptone Broth (10 g/L Tryptone, 5 g/L NaCl). Cells were incubated with penicillin for 1 hour prior to freezing. |
|---|---|
| Grid | Details: R 2/2 copper/rhodium grids, glow discharged |
| Vitrification | Cryogen name: ETHANE-PROPANE MIXTURE / Chamber humidity: 100 % / Instrument: FEI VITROBOT MARK III |
- Electron microscopy
Electron microscopy
| Microscope | FEI POLARA 300 |
|---|---|
| Specialist optics | Energy filter - Name: FEI / Energy filter - Lower energy threshold: 0.0 eV / Energy filter - Upper energy threshold: 20.0 eV |
| Date | Nov 1, 2011 |
| Image recording | Average electron dose: 150 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.2 mm / Nominal defocus min: 10.0 µm / Nominal magnification: 34000 |
| Sample stage | Specimen holder model: OTHER / Tilt series - Axis1 - Min angle: -66.93 ° / Tilt series - Axis1 - Max angle: 59.52 ° |
| Experimental equipment |  Model: Tecnai Polara / Image courtesy: FEI Company |
- Image processing
Image processing
| Details | CTF-corrected. Average number of tilts used in the 3D reconstructions: 128. Average tomographic tilt angle increment: 1. |
|---|---|
| Final reconstruction | Applied symmetry - Point group: C3 (3 fold cyclic) / Resolution.type: BY AUTHOR / Resolution: 30.0 Å / Resolution method: OTHER / Software - Name: IMOD, tomo3D / Number subtomograms used: 96 |
| CTF correction | Details: IMOD |
 Movie
Movie Controller
Controller




 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)