[English] 日本語
 Yorodumi
Yorodumi- EMDB-23947: Bartonella henselae NrnC complexed with pAAAGG in the presence of... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-23947 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Bartonella henselae NrnC complexed with pAAAGG in the presence of Ca2+. D4 Symmetry. | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | RNase / bacteria / enzyme / RNA BINDING PROTEIN / RNA BINDING PROTEIN-RNA complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationnucleobase-containing compound metabolic process / 3'-5' exonuclease activity / nucleic acid binding / metal ion binding Similarity search - Function | |||||||||
| Biological species |  Bartonella henselae (bacteria) / synthetic construct (others) Bartonella henselae (bacteria) / synthetic construct (others) | |||||||||
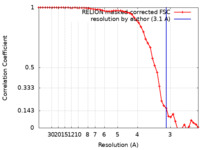
| Method | single particle reconstruction / cryo EM / Resolution: 3.1 Å | |||||||||
 Authors Authors | Lormand JD / Brownfield B | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Elife / Year: 2021 Journal: Elife / Year: 2021Title: Structural characterization of NrnC identifies unifying features of dinucleotidases. Authors: Justin D Lormand / Soo-Kyoung Kim / George A Walters-Marrah / Bryce A Brownfield / J Christopher Fromme / Wade C Winkler / Jonathan R Goodson / Vincent T Lee / Holger Sondermann /   Abstract: RNA degradation is fundamental for cellular homeostasis. The process is carried out by various classes of endolytic and exolytic enzymes that together degrade an RNA polymer to mono-ribonucleotides. ...RNA degradation is fundamental for cellular homeostasis. The process is carried out by various classes of endolytic and exolytic enzymes that together degrade an RNA polymer to mono-ribonucleotides. Within the exoribonucleases, nano-RNases play a unique role as they act on the smallest breakdown products and hence catalyze the final steps in the process. We recently showed that oligoribonuclease (Orn) acts as a dedicated diribonucleotidase, defining the ultimate step in RNA degradation that is crucial for cellular fitness (Kim et al., 2019). Whether such a specific activity exists in organisms that lack Orn-type exoribonucleases remained unclear. Through quantitative structure-function analyses, we show here that NrnC-type RNases share this narrow substrate length preference with Orn. Although NrnC and Orn employ similar structural features that distinguish these two classes of dinucleotidases from other exonucleases, the key determinants for dinucleotidase activity are realized through distinct structural scaffolds. The structures, together with comparative genomic analyses of the phylogeny of DEDD-type exoribonucleases, indicate convergent evolution as the mechanism of how dinucleotidase activity emerged repeatedly in various organisms. The evolutionary pressure to maintain dinucleotidase activity further underlines the important role these analogous proteins play for cell growth. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_23947.map.gz emd_23947.map.gz | 2.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-23947-v30.xml emd-23947-v30.xml emd-23947.xml emd-23947.xml | 18.2 KB 18.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_23947_fsc.xml emd_23947_fsc.xml | 5.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_23947.png emd_23947.png | 128.1 KB | ||
| Filedesc metadata |  emd-23947.cif.gz emd-23947.cif.gz | 5.7 KB | ||
| Others |  emd_23947_additional_1.map.gz emd_23947_additional_1.map.gz emd_23947_half_map_1.map.gz emd_23947_half_map_1.map.gz emd_23947_half_map_2.map.gz emd_23947_half_map_2.map.gz | 6 MB 6.1 MB 6.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-23947 http://ftp.pdbj.org/pub/emdb/structures/EMD-23947 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23947 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23947 | HTTPS FTP |
-Related structure data
| Related structure data |  7mqhMC  7mplC  7mpmC  7mpnC  7mpoC  7mppC  7mpqC  7mprC  7mpsC  7mptC  7mpuC  7mqbC  7mqcC  7mqdC  7mqeC  7mqfC  7mqgC  7mqiC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_23947.map.gz / Format: CCP4 / Size: 10.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_23947.map.gz / Format: CCP4 / Size: 10.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.24 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: #1
| File | emd_23947_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_23947_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_23947_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : NrnC octamer complexed with pAAAGG
| Entire | Name: NrnC octamer complexed with pAAAGG |
|---|---|
| Components |
|
-Supramolecule #1: NrnC octamer complexed with pAAAGG
| Supramolecule | Name: NrnC octamer complexed with pAAAGG / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Molecular weight | Theoretical: 186.621 kDa/nm |
-Supramolecule #2: NrnC octamer
| Supramolecule | Name: NrnC octamer / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Bartonella henselae (bacteria) Bartonella henselae (bacteria) |
-Supramolecule #3: pAAAGG
| Supramolecule | Name: pAAAGG / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2 |
|---|---|
| Source (natural) | Organism:  Bartonella henselae (bacteria) / Synthetically produced: Yes Bartonella henselae (bacteria) / Synthetically produced: Yes |
-Macromolecule #1: NanoRNase C
| Macromolecule | Name: NanoRNase C / type: protein_or_peptide / ID: 1 / Number of copies: 8 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Bartonella henselae (bacteria) Bartonella henselae (bacteria) |
| Molecular weight | Theoretical: 23.446725 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: SMTEIRVHQG DLPNLDNYRI DAVAVDTETL GLQPHRDRLC VVQLSSGDGT ADVIQIAKGQ KSAPNLVRLL SDRDITKIFH FGRFDLAIL AHTFGVMPDV VFCTKIASKL TRTYTDRHGL KEICGELLNV NISKQQQSSD WAAETLSRAQ IEYAASDVLY L HRLKDIFE ...String: SMTEIRVHQG DLPNLDNYRI DAVAVDTETL GLQPHRDRLC VVQLSSGDGT ADVIQIAKGQ KSAPNLVRLL SDRDITKIFH FGRFDLAIL AHTFGVMPDV VFCTKIASKL TRTYTDRHGL KEICGELLNV NISKQQQSSD WAAETLSRAQ IEYAASDVLY L HRLKDIFE ERLKREERES VAKACFQFLP MRANLDLLGW SEIDIFAHS UniProtKB: 3'-5' exonuclease |
-Macromolecule #2: RNA (5'-R(P*AP*AP*AP*GP*G)-3')
| Macromolecule | Name: RNA (5'-R(P*AP*AP*AP*GP*G)-3') / type: rna / ID: 2 / Number of copies: 8 |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 1.633072 KDa |
| Sequence | String: AAAGG |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 Component:
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vitrification | Cryogen name: ETHANE / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TALOS ARCTICA |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller

















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)